Method for preparing epoxybutane
A technology of butylene oxide and hydrogen peroxide, which is applied in organic chemistry and other fields, can solve the problems of low selectivity of butylene oxide and short service life of catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
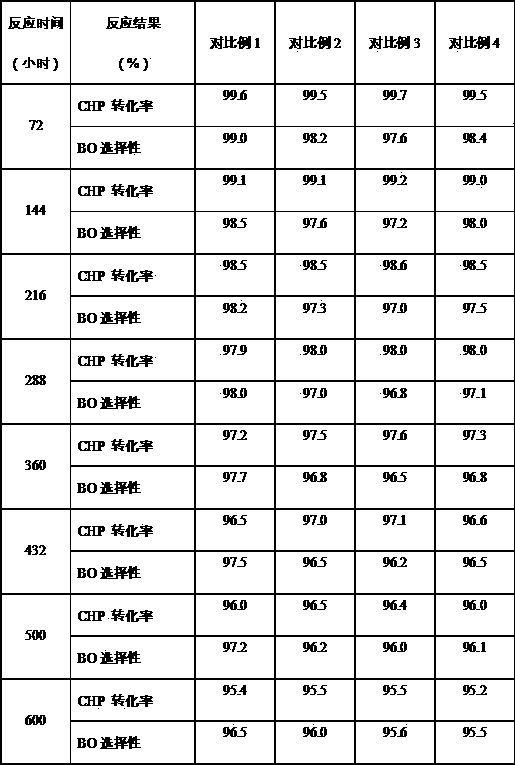
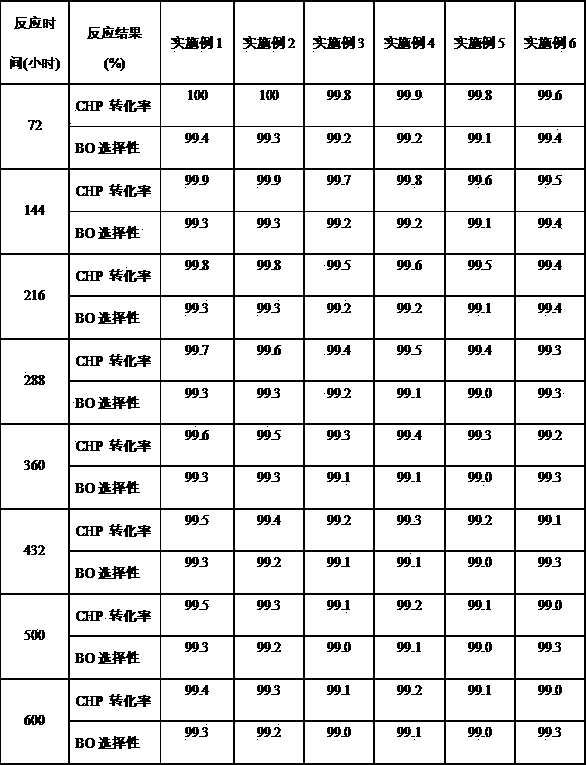
[0036] Under the conditions of 100°C, 0.3 MPa, and the molar ratio of cumene to air at 1:5, cumene and air undergo oxidation reaction to obtain cumene hydroperoxide oxidation solution with a weight concentration of 20-24%.
[0037] Using Na 2 CO 3 Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 5%, wherein the volume ratio of the oxidation solution to the alkali solution is 4:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 4:1. According to the needs of the epoxidation reaction, vacuum enrichment is carried out to it, and the enrichment temperature is 80°C to obtain an oxidizing solution with a concentration of cumene hydroperoxide of 50% by weight. When enriching, the residual water due to washing is also to a certain extent above is removed. After process...
Embodiment 2
[0045] Under the conditions of 98°C, 0.3 MPa, and the molar ratio of cumene to air at 1:5, cumene and air undergo oxidation reaction to obtain a cumene hydroperoxide oxidation solution with a weight concentration of 20-24%.
[0046] Using Na 2 CO 3 Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 5%, wherein the volume ratio of the oxidation solution to the alkali solution is 4:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 4:1. According to the needs of the epoxidation reaction, vacuum enrichment is carried out to it, and the enrichment temperature is 80°C to obtain an oxidizing solution with a concentration of cumene hydroperoxide of 50% by weight. When enriching, the residual water due to washing is also to a certain extent above is removed. After proces...
Embodiment 3
[0054] Under the conditions of 90°C, 0.3 MPa, and the molar ratio of cumene to air at 1:5, cumene and air undergo oxidation reaction to obtain cumene hydroperoxide oxidation solution with a weight concentration of 20-24%.
[0055]Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 2% NaOH, wherein the volume ratio of the oxidation solution to the alkali solution is 4:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 4:1. According to the needs of the epoxidation reaction, vacuum concentration is carried out to it, the concentration temperature is 80°C, and the oxidation solution with a concentration of cumene hydroperoxide of 60% by weight is obtained, and the residual water due to washing with water is also to a certain extent when concentration is carried out. abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com