A method for preparing 13x by using acid leaching slag of laterite nickel ore and bauxite as raw materials
A technology of laterite nickel ore and acid leaching slag, which is applied in the fields of environment, materials and chemical industry, can solve the problem of high raw material prices and achieve the effect of simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
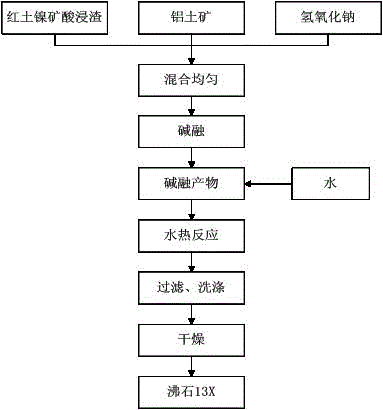
[0019] The acid leaching slag of laterite nickel ore, bauxite and sodium hydroxide were mixed according to the molar ratio of sodium to silicon n(Na 2 O) / n(SiO 2 ) is 1.4:1, the molar ratio of silicon to aluminum n(SiO 2 ) / n(Al 2 o 3 ) is 3:1 and mixed evenly, and reacted with alkali at 650°C for 1h, and then according to the water-sodium molar ratio n(H 2 O) / n(Na 2 O) add water in an amount of 30:1 to obtain a suspension; transfer the obtained suspension to a hydrothermal reaction kettle for crystallization at 60°C for 24 hours, cool to room temperature, filter, wash and dry, and finally obtain a zeolite 13X.
Embodiment 2
[0021] The acid leaching slag of laterite nickel ore, bauxite and sodium hydroxide were mixed according to the molar ratio of sodium to silicon n(Na 2 O) / n(SiO 2 ) is 1.6:1, the molar ratio of silicon to aluminum n(SiO 2 ) / n(Al 2 o 3 ) was 3.5:1 and mixed evenly, and reacted with alkali at 600°C for 2h, and then according to the water-sodium molar ratio n(H 2 O) / n(Na 2 O) add water in an amount of 40:1 to obtain a suspension; transfer the obtained suspension to a hydrothermal reaction kettle for crystallization at 80°C for 12 hours, cool to room temperature, filter, wash and dry, and finally obtain a zeolite 13X.
Embodiment 3
[0023] The acid leaching slag of laterite nickel ore, bauxite and sodium hydroxide were mixed according to the molar ratio of sodium to silicon n(Na 2 O) / n(SiO 2 ) is 2:1, the molar ratio of silicon to aluminum n(SiO 2 ) / n(Al 2 o 3 ) was 3.8:1 and mixed evenly, and reacted with alkali at 550°C for 2h, and then according to the water-sodium molar ratio n(H 2 O) / n(Na 2 O) add water in an amount of 50:1 to obtain a suspension; transfer the obtained suspension to a hydrothermal reaction kettle for crystallization at 90°C for 12 hours, cool to room temperature, filter, wash and dry, and finally obtain a zeolite 13X.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com