Phosphatidase A1 immobilization method
A technology of phospholipase and immobilized phospholipase, which is used in the food and biological fields, and can solve problems such as difficult large-scale application, narrow application range, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
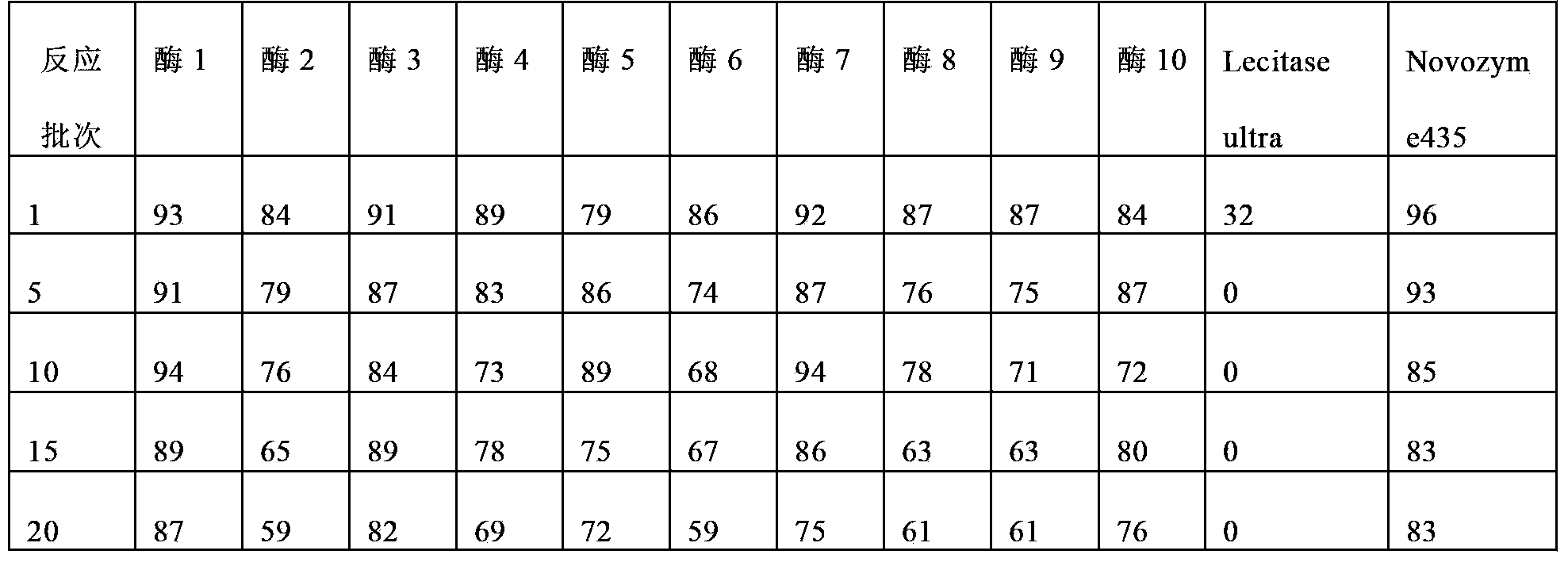
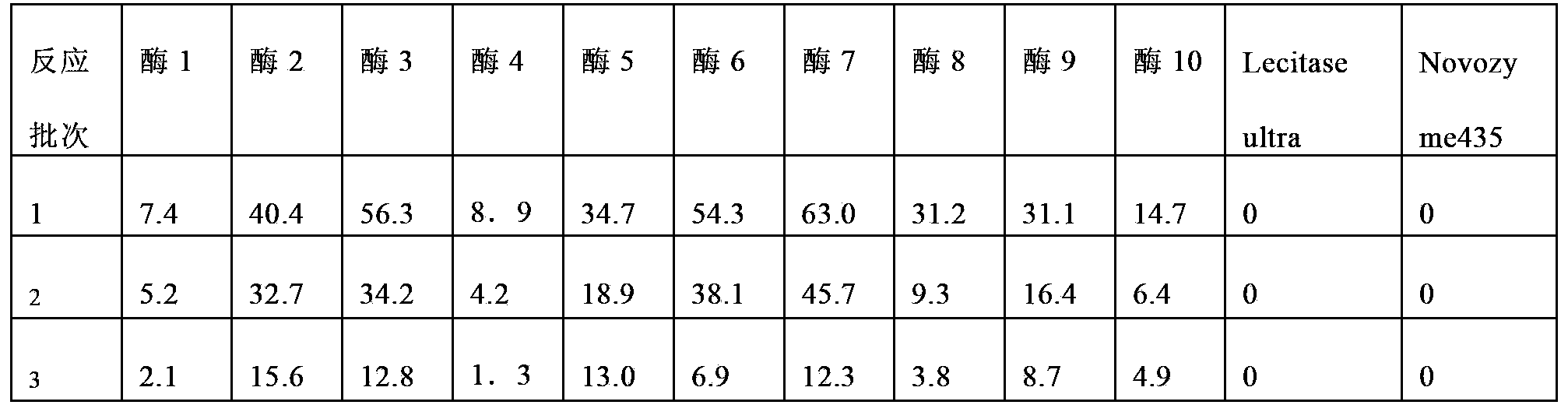
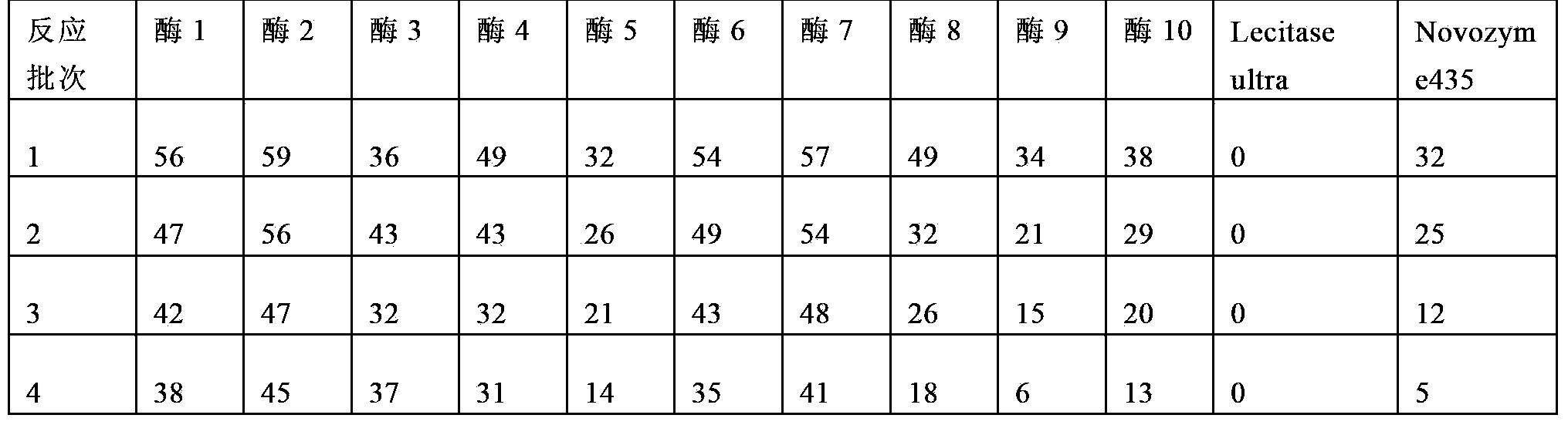
Embodiment 1
[0084] Rinse the D213 carrier with distilled water to remove impurities, soak in 0.5mol / L NaOH solution for 2h, wash with water until neutral, then soak in 0.5mol / L HCl solution for 2h, wash with water until neutral, repeat the above steps 3 times , and finally soaked in 0.5mol / L NaOH solution for 2h, washed with water until neutral.
[0085] Weigh 40g of the processed carrier D21340g into a 250ml conical flask, add 80ml of lecitase Ultra enzyme solution, place it in a shaker at 25°C at 150rpm, shake it at 150rpm, take out the mixture after 1.5h for suction filtration, and fluidize the solid part to a fluidized bed (Midi Glatt) The air inlet temperature is 40°C, the air inlet pressure is 0.6 bar, and dried to a moisture content of about 5%. Labeled Enzyme 1.
Embodiment 2
[0087] Weigh 140 g of the carrier D31 treated according to the carrier treatment method in Example 1 into a 250 ml Erlenmeyer flask, add 80 ml of lecitase Ultra enzyme solution, place it in a shaker at 25°C at 150 rpm, and take out the mixture after 1.5 hours for suction filtration, and remove the solid part Fluidized drying, fluidized bed (Midi Glatt) inlet air temperature 40 ℃, inlet air pressure 0.6 bar, dry to about 5% moisture content. Labeled Enzyme 2.
Embodiment 3
[0089] Weigh 140 g of the carrier D20 treated according to the carrier treatment method in Example 1 into a 250 ml Erlenmeyer flask, add 80 ml of lecitase Ultra enzyme solution, place it in a shaker at 25°C at 150 rpm, and take out the mixture after 1.5 hours for suction filtration. Fluidized drying, fluidized bed (Midi Glatt) inlet air temperature 40 ℃, inlet air pressure 0.6 bar, dry to about 5% moisture content. Labeled Enzyme 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com