A method of preparing estradiol
An estradiol and cell technology, which is applied in the field of using induced mammalian cells to culture in vitro to prepare estradiol, can solve the problems of unpredictable preparation of estrogen, complex regulation network, difficulty in preparation of estrogen, etc., and achieves simple method and by-products. Fewer, milder effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
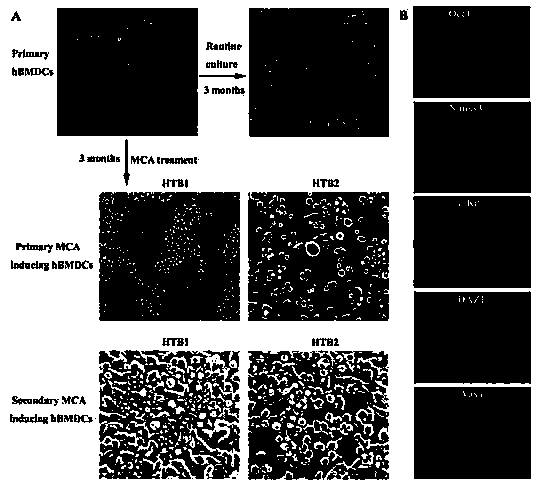
[0044] Human bone marrow-derived cells are used to induce and screen the cells described in the present invention using the compound trimethylcholanthracene (MCA), and use the cells to synthesize sexual estradiol in vitro; the cells have been obtained from the China Center for Type Culture Collection Deposit, deposit number CCTCC C2012173;
[0045] The preparation steps are:
[0046] (1) Human bone marrow-derived cells (HMBDCs, mainly composed of human bone marrow mesenchymal stem cells) were prepared by primary culture using existing technology (this example uses bone marrow flushing and adherence method), and the prepared cells were cultured in a medium containing 15% fetal DMEM medium with bovine serum, cultured at 37°C, 5% CO 2 , up to 10 5 cells / flask;
[0047] (2) Add MCA to the medium to make the final concentration 1ug / ml, and replace the medium containing MCA twice a week; MCA treatment ends after 1 week; cells are kept at 37°C, 5% CO 2 environment, continuous rou...
Embodiment 2
[0055] After induction by long-term culture in vitro using mouse bone marrow-derived cells (mBMDCs), the cells described in the present invention are produced and screened out, and the cells are used to synthesize sexual estradiol in vitro; the specific steps are:
[0056] (1) Isolate and purify mouse bone marrow-derived cells (mBMDCs), and continuously subculture them in vitro. After about 20 passages or about 6 consecutive cultures, the induced mBMDCs have the ability to produce estradiol;
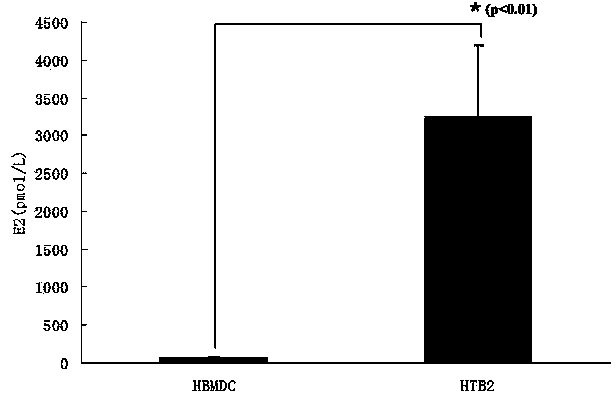
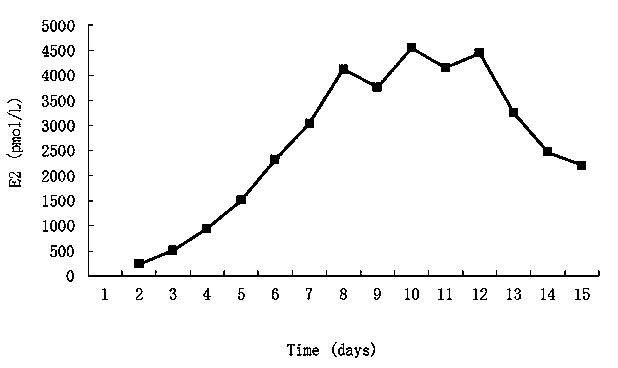
[0057] (2) The concentration of estradiol produced by cells after the induction can reach up to 5000 pmol / L.
Embodiment 3
[0059] Mouse embryonic fibroblasts are used to induce the pluripotency genes Oct4, Sox2, Nanog, and c-KIT in vitro to produce and screen out the cells described in the present invention, and use the cells to synthesize estradiol in vitro; The steps are:
[0060] (1) Isolate and culture mouse embryonic fibroblasts;
[0061] (2) Using the method of gene introduction, transfer the pluripotency genes Oct4, Sox2, Nanog, c-KIT, screen the obtained cells, select the cells that can produce a higher concentration of estradiol, and proliferate and culture;
[0062] (3) The concentration of estradiol produced by the cells after the above induction can reach about 3000pmol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com