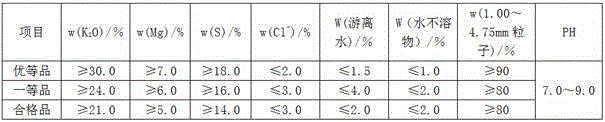
Production method of potash magnesium sulfate fertilizer
A technology of potassium magnesium sulfate fertilizer and production method, which is applied in the production field of potassium magnesium sulfate fertilizer, can solve problems such as excessive chloride ions and failure to meet the quality requirements of potassium magnesium sulfate fertilizer, and achieve low production cost, guaranteed quality, and market source wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
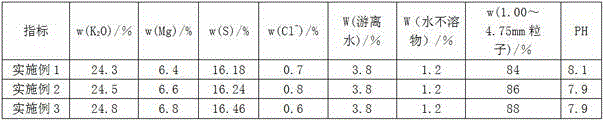
Embodiment 1
[0028] (1) Preparation of potassium bisulfate slurry:
[0029] Add 108.9Kg concentrated sulfuric acid (H 2 SO 4 mass content 90%), stir and heat the reactor to 80°C, then add 149Kg potassium chloride solution (KCl mass content 50%) into the reactor, keep the temperature in the reactor at 80°C and continue stirring for 30min to make the hydrogen chloride gas completely Escape, obtain potassium bisulfate slurry 221.4Kg (KHSO 4 mass content 61.4%).
[0030] (2) Preparation of magnesium oxide slurry:
[0031] Add 40Kg of magnesia and 20Kg of clear water into a plastic bucket, and stir to prepare 60Kg of magnesia slurry for use (66.7% MgO mass content).
[0032] (3) Neutralization reaction:
[0033] Add the magnesium oxide slurry prepared in (2) to the potassium bisulfate slurry obtained in (1), heat the temperature of the reaction kettle to 120°C, and continue stirring for 5-10 minutes to obtain the potassium magnesium sulfate slurry.
[0034] (4) Cooling, crushing and drying:...
Embodiment 2
[0037] (1) Preparation of potassium bisulfate slurry:
[0038] Add 105.4Kg concentrated sulfuric acid (H 2 SO 4 mass content 93%), stir and heat the reactor to 120°C, then add 120.2Kg potassium chloride solution (KCl mass content 62%) into the reactor, keep the temperature in the reactor at 120°C and continue to stir for 45min to make hydrogen chloride gas All escape, obtain potassium bisulfate slurry 189.1Kg (KHSO 4 mass content 71.9%).
[0039] (2) Preparation of magnesium oxide slurry:
[0040] Add 40Kg of magnesia and 40Kg of clear water into a plastic bucket, stir to prepare 80Kg of magnesia slurry for use (MgO mass content 50%).
[0041] (3) Neutralization reaction:
[0042] Add the magnesium oxide slurry prepared in (2) to the potassium hydrogen sulfate slurry obtained in (1), heat the temperature of the reaction kettle to 140°C, and continue stirring for 5-10 minutes to obtain the potassium magnesium sulfate slurry.
[0043] (4) Cooling, crushing and drying:
[...
Embodiment 3
[0046] (1) Preparation of potassium bisulfate slurry:
[0047] Add 123.8Kg concentrated sulfuric acid (H 2 SO 4 mass content 95%), stir and heat the reactor to 140°C, then add 106.4Kg potassium chloride solution (KCl mass content 70%) into the reactor, keep the temperature in the reactor at 140°C and continue stirring for 60min to make hydrogen chloride gas All escape, obtain potassium bisulfate slurry 193.7Kg (KHSO 4 mass content 70.2%).
[0048] (2) Preparation of magnesium oxide slurry:
[0049] Add 40Kg of magnesia and 80Kg of clear water into a plastic bucket, stir and prepare to obtain 120Kg of magnesia slurry for use (MgO mass content 33.2%).
[0050] (3) Neutralization reaction:
[0051] Add the magnesium oxide slurry prepared in (2) to the potassium bisulfate slurry obtained in (1), heat the temperature of the reaction kettle to 160°C, and continue stirring for 5-10 minutes to obtain the potassium magnesium sulfate slurry.
[0052] (4) Cooling, crushing and dryi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com