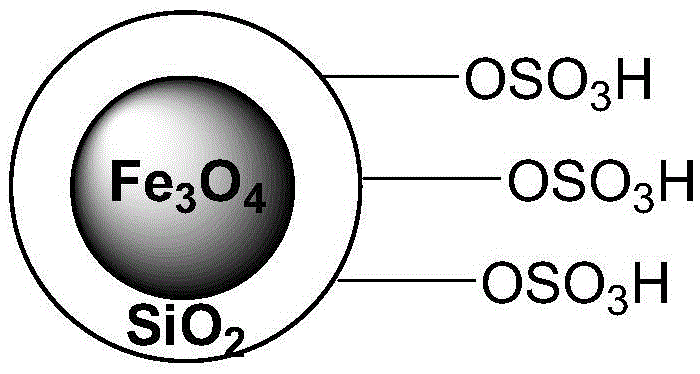
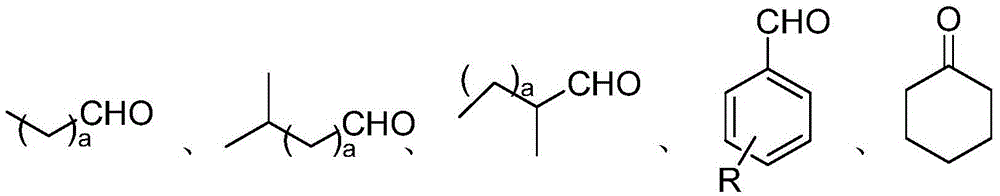
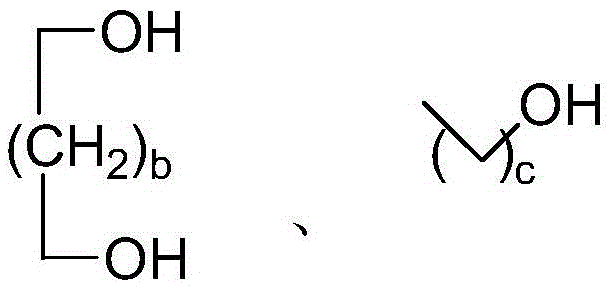A method for preparing acetal (ketone) catalyzed by an acidic magnetic material containing -so3h
A technology of -SO3H and magnetic materials, applied in the preparation of organic compounds, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of high catalyst preparation price, large catalyst usage, post-treatment Complex process and other issues, to achieve the effect of easy industrialized large-scale production, poor miscibility, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: 10 mmol of n-butyraldehyde, 10 mmol of 1,3-propanediol and 2.72 g of acidic magnetic material were added to a 25 ml single-necked bottle with a stirring bar and a condenser. The reaction was vigorously stirred at 110° C. for 0.75 h, cooled to room temperature after the reaction, and the catalyst was sucked out with a magnet. The conversion rate of n-butyraldehyde was 97% as detected by the gas chromatography of the reaction liquid, the selectivity of the product n-butyraldehyde 1,3-propanediol acetal was 100%, and the calculated yield was 97%. The catalyst was recycled without treatment.
Embodiment 2
[0024] Example 2: 10mmol of benzaldehyde, 15mmol of 1,3-propanediol and 3.04g of acidic magnetic material were added to a 50ml single-necked bottle with a stirring bar and a condenser. The reaction was vigorously stirred at 110° C. for 1.5 h, cooled to room temperature after the reaction, and the catalyst was sucked out with a magnet. The conversion rate of benzaldehyde was 93% as detected by the gas chromatography of the reaction liquid, the selectivity of the product benzaldehyde 1,3-propanediol acetal was 100%, and the calculated yield was 93%. The catalyst was recycled without treatment.
Embodiment 3
[0025] Example 3: 10mmol of p-nitrobenzaldehyde, 25mmol of ethylene glycol and 2.41g of acidic magnetic material were added to a 50ml single-necked bottle with a stirring bar and a condenser. The reaction was vigorously stirred at 110° C. for 2 h, cooled to room temperature after the reaction, and the catalyst was sucked out with a magnet. Reaction liquid gas chromatography detects that the conversion rate of p-nitrobenzaldehyde is 96%, and the selectivity of product p-nitrobenzaldehyde-ethylene glycol acetal is 100%, and its productive rate is calculated as 96%, and the catalyzer is circulated without treatment use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com