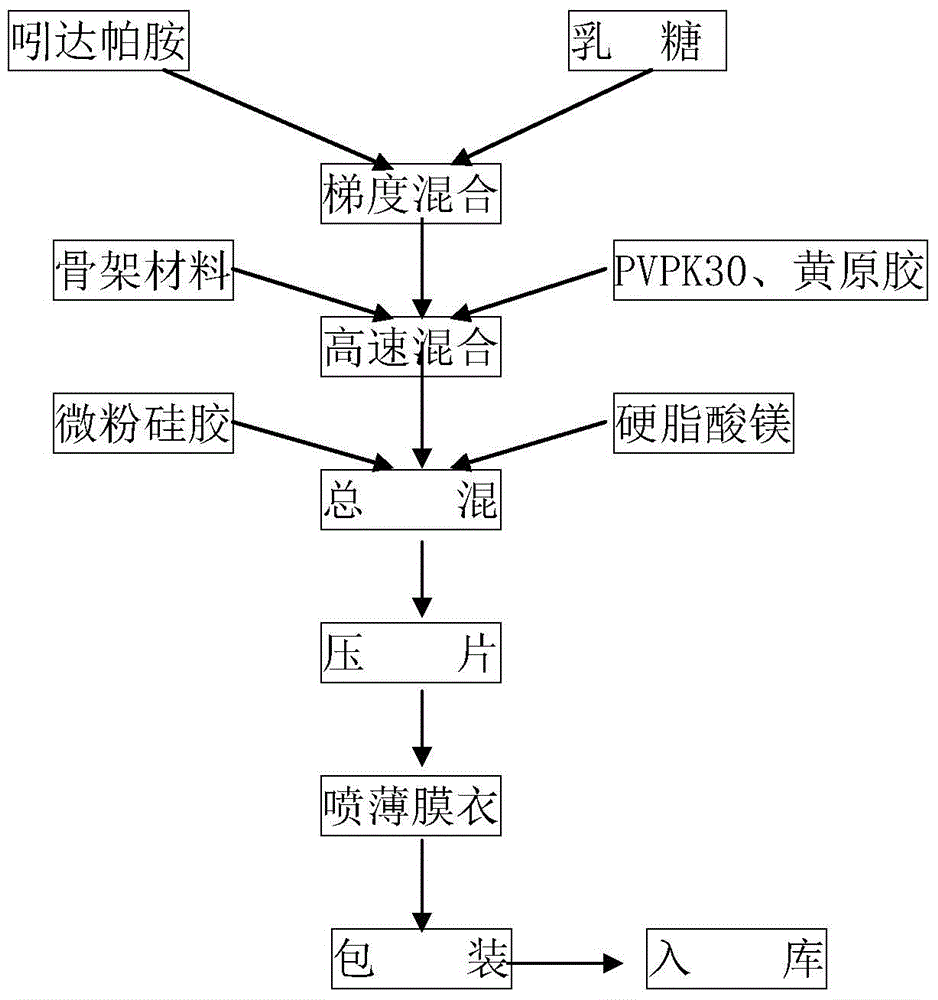
Indapamide slow release medicine containing composite lactose
A technology for indapamide and sustained-release drugs, applied in the field of medicine, can solve the problems of large limitations, high cost, poor mixing uniformity, etc., and achieve the effects of improving production efficiency, reducing production energy consumption, and achieving good uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
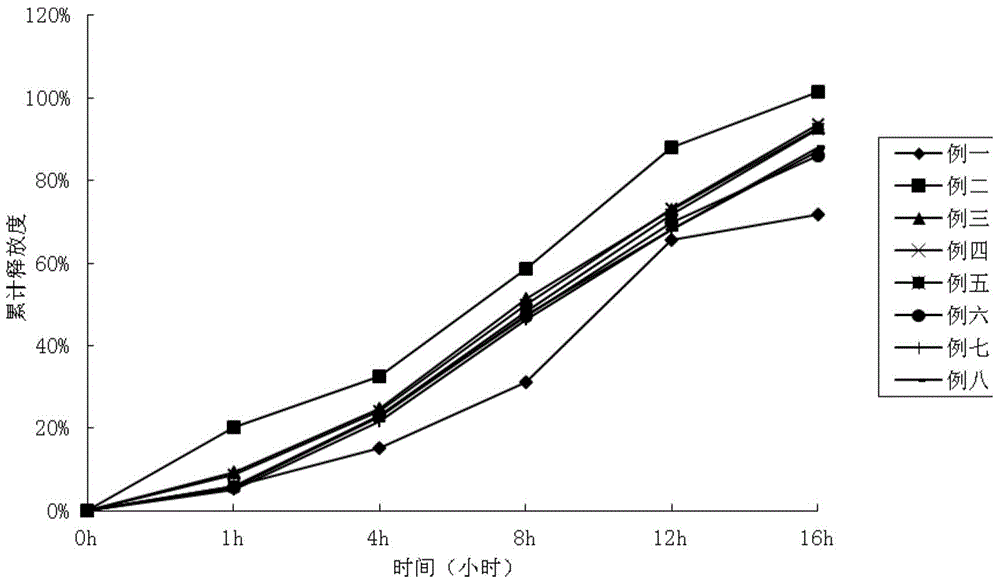
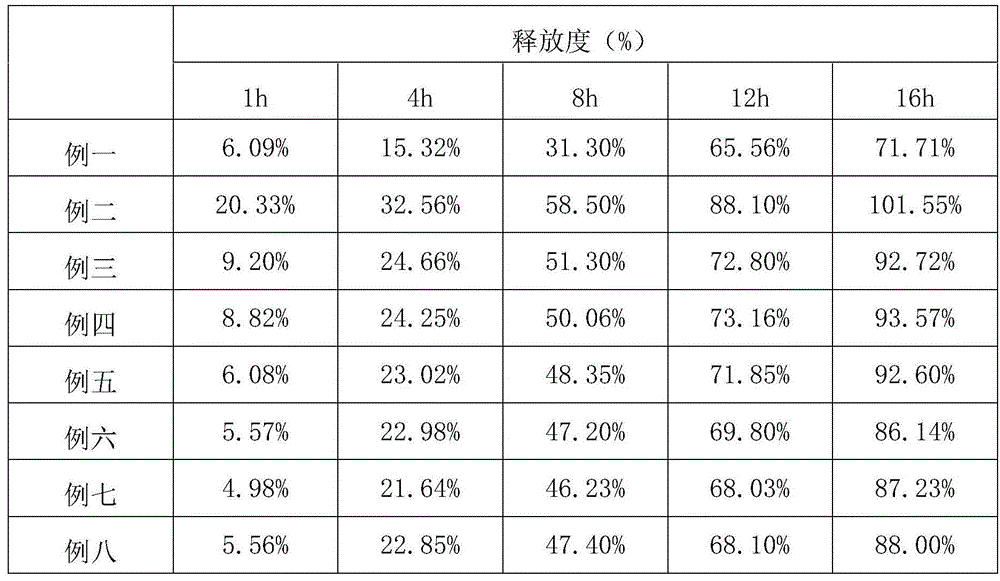
Examples
Embodiment 1
[0035] In the first embodiment, the skeleton material is hydroxypropyl methylcellulose K4M, and the lubricant is 0.3% silica gel and 0.75% magnesium stearate;
Embodiment 2
[0036] In the second embodiment, the skeleton material is hydroxypropyl methylcellulose K4M, and the lubricant is 0.3% silica gel and 0.75% magnesium stearate;
Embodiment 3
[0037] In the third embodiment, the skeleton material is hydroxypropyl methylcellulose K4M, and the lubricant is 0.3% silica gel and 0.75% magnesium stearate;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com