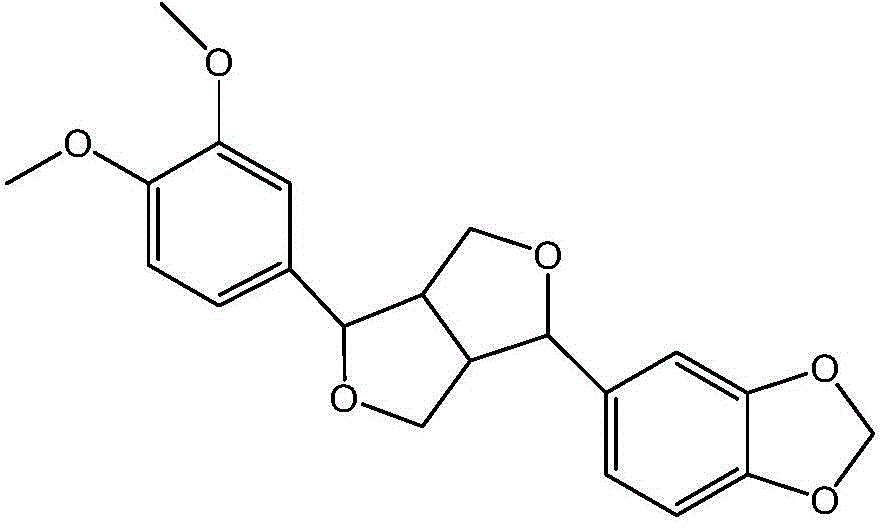
Use of fargesin and its derivative in preparation of drugs for treating or preventing pulmonary hypertension
A technology for pulmonary arterial hypertension and octopylin, which is applied in the directions of drug combinations, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
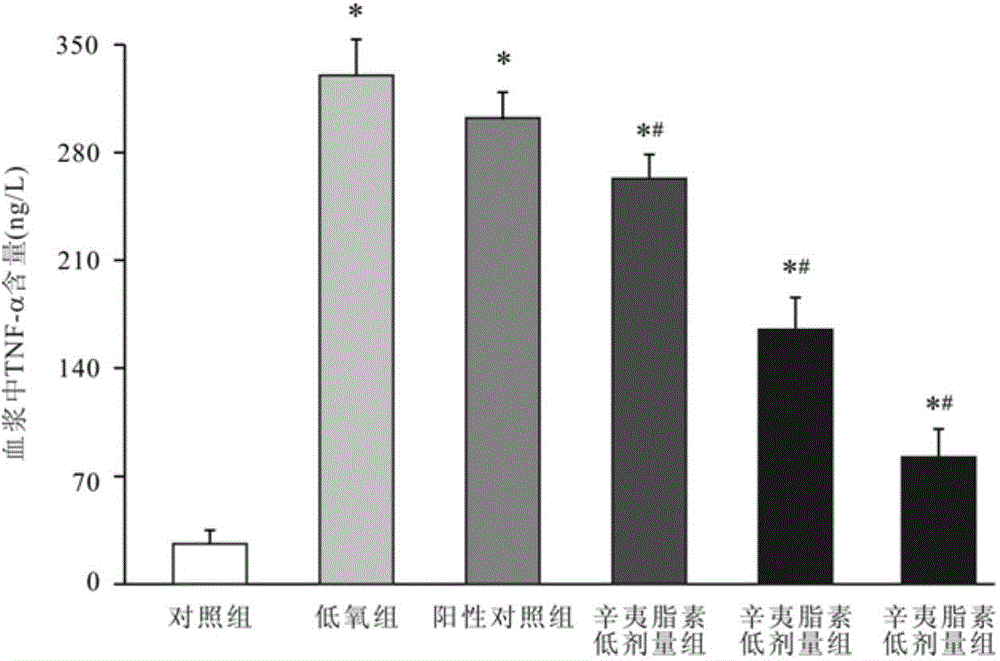
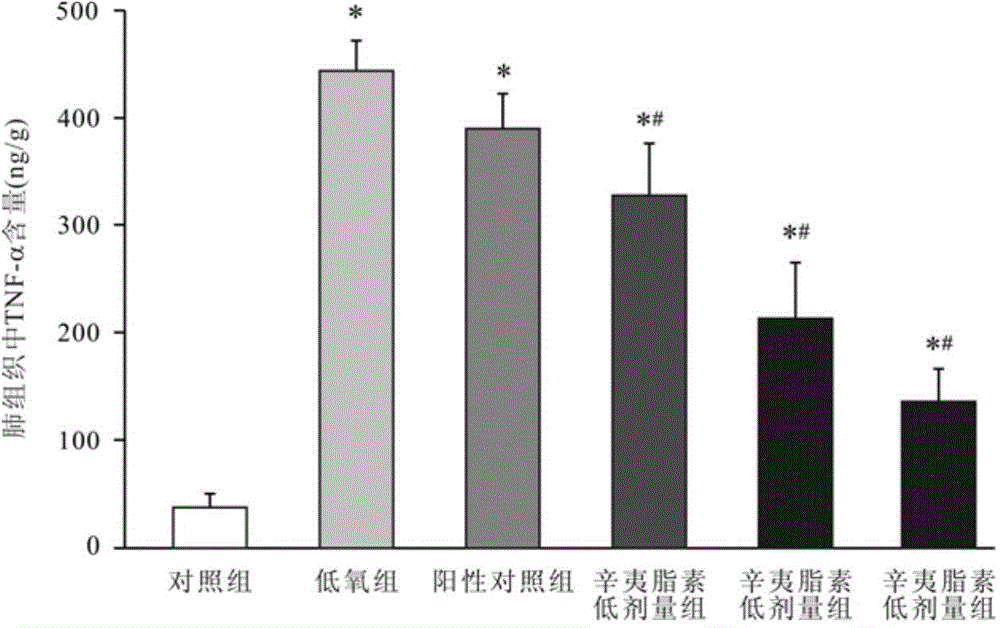
Image
Examples
Embodiment 1
[0031] Make magnolipin slow-release tablet according to the following formula (all used contents are weight percentages)
[0032]
[0033]
[0034] The preparation method is as follows:
[0035] (1) Mix magnolipin, lactose, hydroxypropyl methylcellulose, microcrystalline cellulose, and polyvinylpyrrolidone evenly, and granulate with absolute ethanol;
[0036] (2) Put the wet granules in a drying oven at about 50°C, and control the moisture content to about 2%;
[0037] (3) After the dried granules are sized through a 20-mesh sieve, magnesium stearate is added, mixed evenly, and pressed into tablets according to the specifications.
Embodiment 2
[0039] Make xinyisu tablet according to following formula (contents used are weight percentages)
[0040]
[0041] The preparation method is as follows:
[0042] (1) Pulverize magnoliatrin and lactose respectively, pass through a 100-mesh sieve, and directly pass the starch through a 100-mesh sieve for subsequent use;
[0043] (2) Take an appropriate amount of starch and add an aqueous solution to prepare a starch paste for use as a binder;
[0044] (3) Mix magnoliatin, lactose and the remaining amount of starch, add a binder to make a soft material, and pass through a 20-mesh sieve to granulate;
[0045] (4) Place the wet granules in a drying oven at about 50°C, and control the moisture content to about 2%;
[0046] (5) After the dry granules are sized through a 20-mesh sieve, magnesium stearate is added, mixed evenly, and pressed into tablets according to the specifications.
Embodiment 3
[0048] Make magnolipin dispersible tablet according to the following formula (all contents used are weight percentages)
[0049]
[0050]
[0051] The preparation method is as follows:
[0052] (1) By magnesia, microcrystalline cellulose, sodium carboxymethyl starch, aspartame, cross 80 mesh sieves, for subsequent use;
[0053] (2) Take the full amount of polyvinylpyrrolidone K30, make an aqueous solution with a concentration of 5%, stir until it is completely dissolved, and prepare it as an adhesive;
[0054] (3) Mix magnolipin, microcrystalline cellulose, sodium carboxymethyl starch, and aspartame evenly, make a soft material with a binder, and pass through a 20-mesh sieve to granulate;
[0055] (4) Dry the wet granules at about 60°C, and control the moisture to about 2%
[0056] (5) After the dried granules are sized through a 20-mesh sieve, add talcum powder and magnesium stearate, mix well, and press into tablets according to the specifications.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com