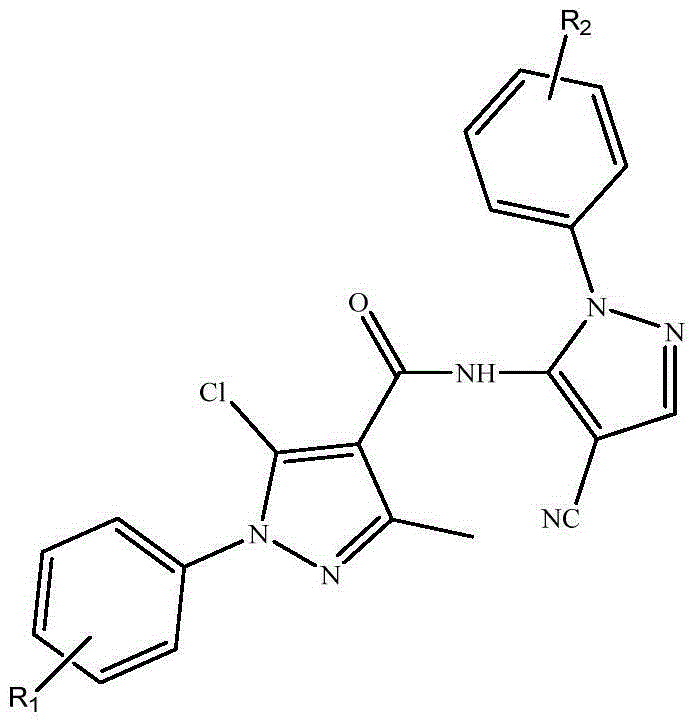
Preparation and application of bispyrazole carboxamide derivative to the control of rice black streaked dwarf disease
A technology of bispyrazole amides and derivatives, applied in the field of bispyrazole amide compounds, can solve problems such as few reports of antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: the synthesis of compound 1:
[0020]
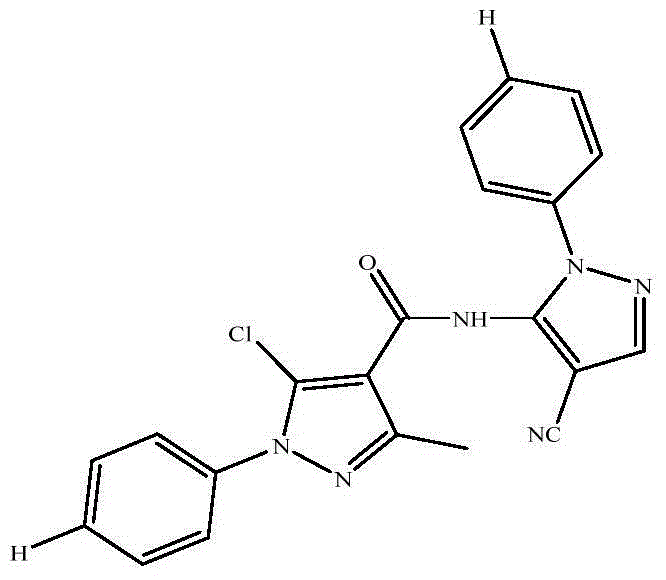
[0021] In the three-necked flask, add 30mL of water and 3.6g (0.025mol) of phenylhydrazine hydrochloride, stir well at room temperature, slowly add an appropriate amount of sodium carbonate to adjust the pH of the system to 7-8, and then dropwise add ethyl acetoacetate dissolved in 10mL of ethanol 3.3 mL (0.026 mol) of the ester was heated slowly to 65° C. under a nitrogen-tight condition and stirred for 5 h. The reaction was traced by TLC. After the reaction was completed, it was cooled, and the crude product a was obtained by suction filtration. DMF and POCl 3 Mix in an ice bath for 30 minutes, then dissolve the above solid a in the above mixture, and heat to 70-80°C for 5 hours. After cooling to room temperature, pour into ice water, filter with suction and wash with water, and dry to obtain solid b. Dissolve solid b in water, heat to 70-80°C, add potassium permanganate solution dropwise under stirring, con...
Embodiment 2
[0024] Embodiment two: the synthesis of compound 2:
[0025]
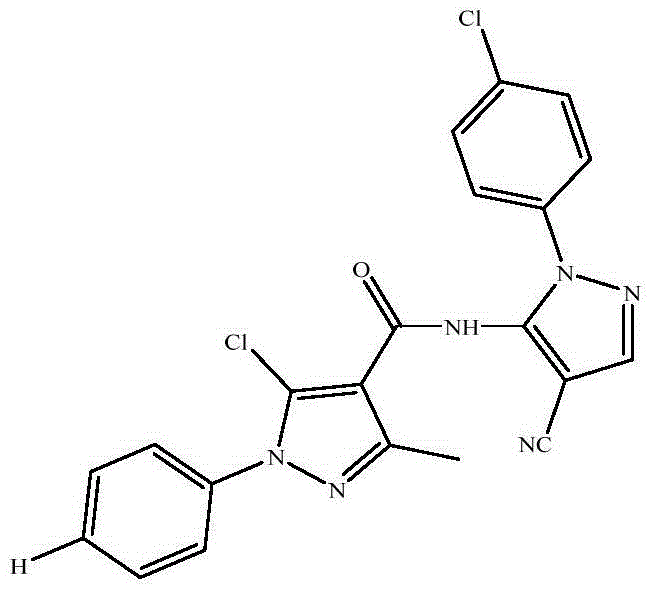
[0026] Synthetic method is the same as embodiment one. Step 4 is synthesized by replacing phenylhydrazine hydrochloride with p-chlorophenylhydrazine hydrochloride. The target compound was obtained as white crystals. Yield 78%, m.p.171-172°C; 1 H NMR (400MHz, CDCl 3 )δ8.12(s,1H),7.55-7.43(m,9H),2.65(s,3H).MS(ESI): 438.68(C 21 h 14 Cl 2 N 6 O,[M+H] + ).Anal.Calcd for C 21 h 14 Cl 2 N 6 O: C, 57.68; H, 3.23; N, 19.22; Found: C, 57.72; H, 3.81; N, 21.11.
Embodiment 3
[0027] Embodiment three: the synthesis of compound 3:
[0028]
[0029] Synthetic method is the same as embodiment one. Step 4 is synthesized by replacing phenylhydrazine hydrochloride with p-methylphenylhydrazine hydrochloride. The target compound was obtained as white crystals. Yield 62%, m.p.123-125℃; 1 H NMR (400MHz, CDCl 3 )δ8.12(s,1H),7.63-7.41(m,9H),2.66(s,3H),2.42(s,3H).MS(ESI):418.02(C 22 h 17 ClN 6 O,[M+H] + ).Anal.Calcd for C 22 h 17 ClN 6 O: C, 63.39; H, 4.11; N, 20.16; Found: C, 63.71; H, 4.42; N, 21.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com