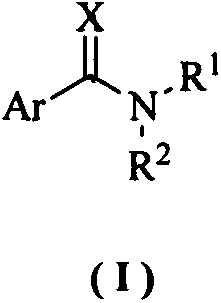
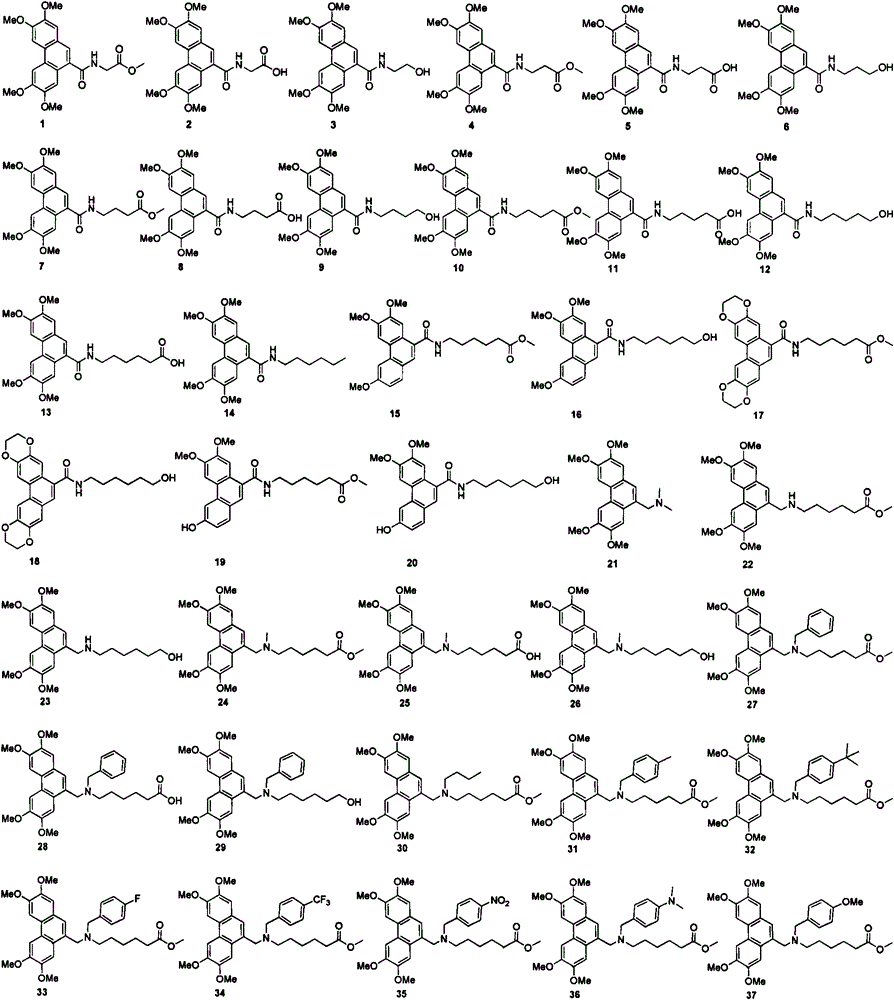
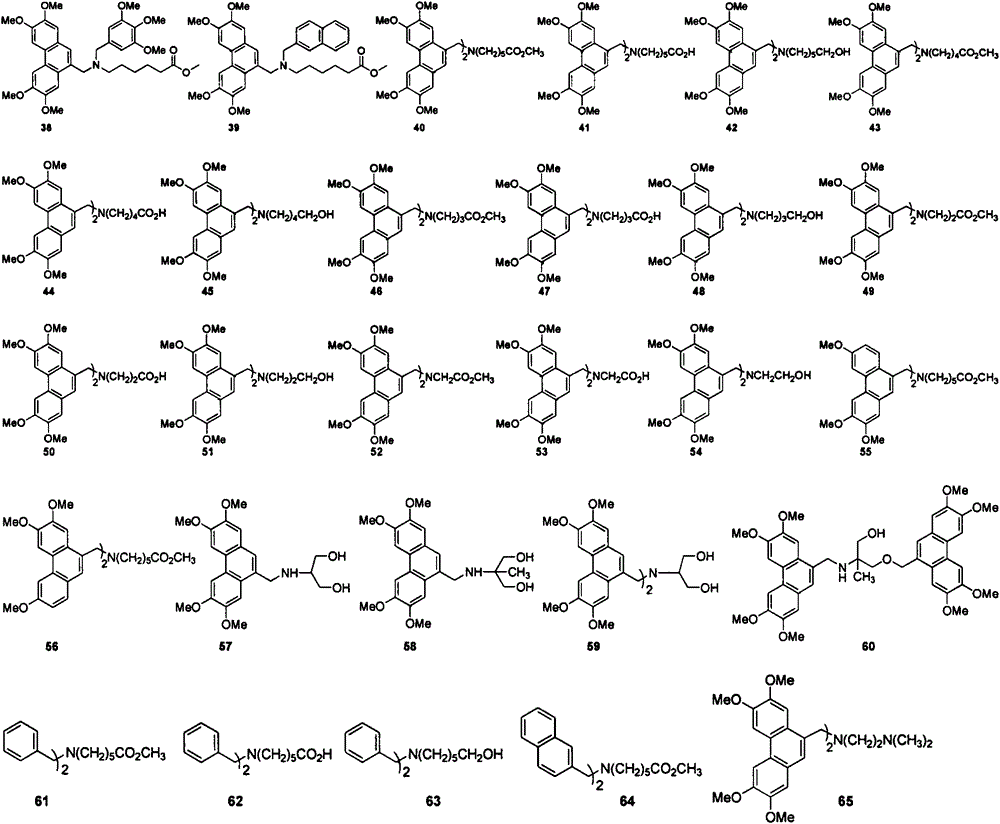
Aryl methylamine compound and preparation method thereof and application in resisting plant virus
An anti-plant virus agent, arylmethylamine technology, applied in the field of pesticides, can solve problems such as reducing the severity of symptoms, and achieve the effects of low toxicity, significant anti-plant virus activity, and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of aromatic methylamine 1-3:
[0026]
[0027] Synthesis of 2,3,6,7-tetramethoxy-9-phenanthroyl chloride (67)
[0028] Add 0.80g (2.34mmol) of 2,3,6,7-tetramethoxy-9-phenanthrene acid (66) and 20mL oxalyl chloride into a 100mL single-necked bottle; add 2 drops of DMF in a water bath; then naturally rise to room temperature, After reacting for another 3 h, the oxalyl chloride was evaporated under normal pressure to obtain 0.82 g of yellow solid 67; it was directly used in the next reaction without further treatment.
[0029] Synthesis of Methyl 2-(N-(2,3,6,7-tetramethoxy-9-phenanthrene)amino)acetate (1)
[0030] Add 0.24g glycine methyl ester hydrochloride, 25mL CH 2 Cl 2 , 0.34g Et 3 N, slowly dropwise add 0.40g of 2,3,6,7-tetramethoxy-9-phenanthroyl chloride (67) in CH 2 Cl 2 The solution was 20 mL, and after the dropwise addition was completed, it was slowly raised to room temperature and reacted for 2 hours, and TLC detected that ...
Embodiment 2
[0035] Embodiment 2: the synthesis of aromatic methylamine 22-26:
[0036]
[0037] Synthesis of 2,3,6,7-tetramethoxy-9-phenanthrenemethanol (68)
[0038] Add 1.03g of 2,3,6,7-tetramethoxy-9-phenanthroic acid (66) and 20mL THF into a 100mL single-necked bottle; add 0.28g of LiAlH in batches under cooling in an ice-water bath 4 , After the addition is complete, heat to reflux for 1.5h, then cool naturally to room temperature. Add 20 mL of CH to the reaction solution 2 Cl 2 , and then slowly add 1mol / L HCl dropwise in an ice-water bath until the white flocculent precipitate disappears; 2 Cl 2 (10mL×3) extraction; combined organic phase, washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 dry. Filtrate under reduced pressure and then concentrate to obtain a solid, which is then recrystallized with ethyl acetate to obtain a white solid with a yield of 95.0% and a melting point of 183-185°C. 1 H NMR (400MHz, CDCl 3 )δ7.83(s, 1H), 7.78(s, 1H), 7.59(s, 1H), 7....
Embodiment 3
[0051] Embodiment 3: the synthesis of arylmethylamine 4-21 and 27-65: (synthetic steps are with reference to embodiment 1 and 2, data are as follows)
[0052] Synthesis of methyl 3-(N-(2,3,6,7-tetramethoxy-9-phenanthroyl)amino)propionate (4)
[0053] White solid, yield: 90.9%, melting point: 171-172°C. 1 H NMR (400MHz, CDCl 3 )δ7.88(s, 1H), 7.78(s, 1H), 7.74(s, 1H), 7.70(s, 1H), 7.20(s, 1H), 6.75(t, J=5.6Hz, 1H), 4.13(s, 6H), 4.03(s, 6H), 3.86-3.82(m, 2H), 3.73(s, 3H), 2.77(t, J=5.6Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ173.1, 170.1, 150.2, 149.3, 148.9, 129.9, 125.5, 125.0, 124.7, 124.0, 123.1, 108.6, 106.3, 102.7, 102.5, 56.0, 56.0, 55.9, 55.9, 51.9, 35.9, 34. (m / z): calcd.for C 23 h 26 NO 7 [M+H] + 428.1704; found 428.1707.
[0054] Synthesis of 3-(N-(2,3,6,7-tetramethoxy-9-phenanthroyl)amino)propionic acid (5)
[0055] White solid, yield: 86.2%, melting point: 222-224°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.27(s, 1H), 8.59(t, J=5.2Hz, 1H), 8.04(s, 1H), 8.01(s, 1H), 7.73...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com