Method for simultaneously preparing potassium nitrate and calcium chloride from calcium nitrate solution and potassium chloride
A technology of potassium chloride and calcium nitrate, applied in the preparation of alkali metal nitrate, calcium/strontium/barium chloride, calcium/strontium/barium halide, etc., can solve the problems of low product yield and low purity of by-products, etc. Achieve the effect of high product purity, improved separation effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
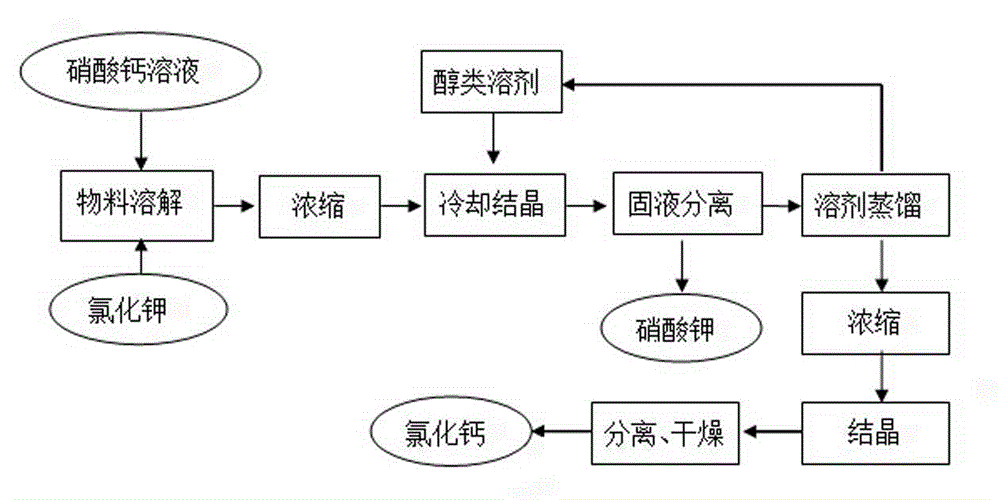
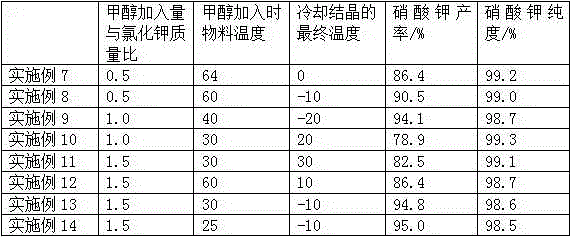
[0018] Prepare 1640g concentration and be that the calcium nitrate solution of 30% joins in the stainless steel reactor of belt coil heating and jacket cooling, then adds 447g potassium chloride solid in calcium nitrate solution, is heated to potassium chloride solid and dissolves completely, then The mixed solution is concentrated, and when the surface of the mixed solution just appears crystallization, that is, when it reaches a saturated state, stop heating, and control the temperature of the material through the jacket cooling water. When the temperature drops to 58°C, add 223.5g of methanol (equivalent to 50% of the mass of potassium chloride), continue to cool down and crystallize under stirring conditions, and continue to stir for 60 minutes to ensure that potassium nitrate crystals are fully separated. Potassium nitrate solution washes the solid, then the solid is dried to obtain 436g of potassium nitrate pure product, the yield is 72%, and the test content is 99.2%. Th...
Embodiment 2
[0021] Prepare 1640g concentration and be that the calcium nitrate solution of 30% joins in the stainless steel reactor of belt coil heating and jacket cooling, then adds 447g potassium chloride solid in calcium nitrate solution, is heated to potassium chloride solid and dissolves completely, then The mixed solution is concentrated, and when the surface of the mixed solution just appears crystallization, that is, when it reaches a saturated state, stop heating, and control the temperature of the material through the jacket cooling water. When the temperature drops to 50°C, add 330g of methanol (equivalent to chlorine 74% of the mass of potassium nitrate), continue to cool down and crystallize under stirring conditions, continue to stir for 180 minutes to ensure that potassium nitrate crystals are fully precipitated, control the temperature through the freezing system and when the temperature drops to -20°C, all crystallized materials are precipitated, and the solids are pumped ...
Embodiment 3
[0023] Prepare 1640g concentration and be that the calcium nitrate solution of 30% joins in the stainless steel reactor of belt coil heating and jacket cooling, then adds 447g potassium chloride solid in calcium nitrate solution, is heated to potassium chloride solid and dissolves completely, then The mixed solution is concentrated, and when the surface of the mixed solution just appears crystallization, that is, when it reaches a saturated state, stop heating, and control the temperature of the material through the jacket cooling water. When the temperature drops to 50°C, add 223.5g of methanol (equivalent to 50% of the mass of potassium chloride), continue to cool down and crystallize under stirring conditions, continue to stir for 30 minutes to ensure that potassium nitrate crystals are fully precipitated, control the temperature through the freezing system and when the temperature drops to -10°C, all crystallized materials are precipitated, and the solid Separation by sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com