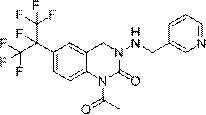
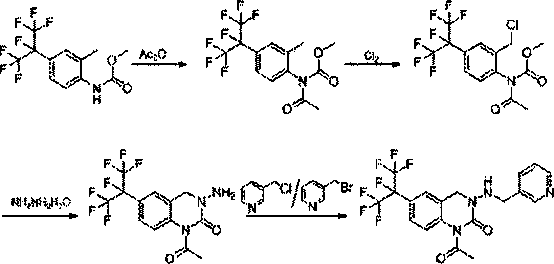
Preparation method of pyridylquinazoline
A technology of pyridine quinazoline and halomethylpyridine, which is applied in the field of preparation of pyridine quinazoline, can solve problems such as difficulty in industrial production, 3-pyridine formaldehyde is not suitable for storage, etc., so as to reduce raw material cost, production cost, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 66.9g (0.20mol) of 2-methyl-4-(perfluoropropan-2-yl)phenyl carbamate, 12.9g of sodium methoxide (0.24mol), and 330g of toluene into the A 500mL three-neck flask with a thermometer, after reflux for 0.5 hours, added 24.5g (0.24mol) acetic anhydride, and then refluxed for 4 hours. After the reaction was completed, the sodium carbonate aqueous solution was washed to neutrality, and the water layer was separated, and the oil layer was distilled under reduced pressure to obtain 71.5 g methyl acetyl (2-methyl-4-(perfluoropropan-2-yl)-phenyl) carbamate, content 95.5% (LCD external standard), yield 91%.
[0027] Add 71.5g (0.182mol) of methylacetyl (2-methyl-4-(perfluoropropan-2-yl)-phenyl) carbamate and 285g of chlorobenzene into the 1. A 1000mL three-necked flask with a ventilation device, control the reaction temperature at 55°C, feed 15.5g (0.218mol) chlorine gas, control the feed time for 10h, and continue the reaction for 2h. After the reaction is completed, wash the ...
Embodiment 2
[0031] Add 66.9g (0.20mol) of 2-methyl-4-(perfluoropropan-2-yl)phenyl carbamate, 12.9g of sodium methoxide (0.24mol), and 400g of toluene into the A 500mL three-neck flask with a thermometer, after reflux for 0.5 hours, added 24.5g (0.24mol) acetic anhydride, and then refluxed for 4 hours. After the reaction was completed, the sodium carbonate aqueous solution was washed to neutrality, and the water layer was separated, and the oil layer was distilled under reduced pressure to obtain 71.5 g methyl acetyl (2-methyl-4-(perfluoropropan-2-yl)-phenyl) carbamate, content 95.8% (LC external standard), yield 91.3%.
[0032]Add 71.5g (0.183mol) of methylacetyl (2-methyl-4-(perfluoropropane-2-yl)-phenyl) carbamate and 285g of chlorobenzene into the 1. A 1000mL three-necked flask with a ventilation device, control the reaction temperature at 60°C, feed 15.6g (0.220mol) chlorine gas, control the feeding time for 10h, and continue the reaction for 2h. After the reaction is completed, wash ...
Embodiment 3
[0036] Add 66.9g (0.20mol) of 2-methyl-4-(perfluoropropan-2-yl)phenylcarbamate, 12.9g of sodium methoxide (0.24mol), and 465g of toluene into the A 500mL three-neck flask with a thermometer, after reflux for 0.5 hours, added 24.5g (0.24mol) acetic anhydride, and then refluxed for 4 hours. After the reaction was completed, the sodium carbonate aqueous solution was washed to neutrality, and the water layer was separated, and the oil layer was distilled under reduced pressure to obtain 71.5 g methylacetyl (2-methyl-4-(perfluoropropan-2-yl)-phenyl) carbamate, content 95.5% (LC external standard), yield 91%.
[0037] Add 71.5g (0.182mol) of methylacetyl (2-methyl-4-(perfluoropropane-2-yl)-phenyl) carbamate and 430g of chlorobenzene into the 1. A 1000mL three-necked flask with a ventilation device, control the reaction temperature at 55°C, feed 15.5g (0.218mol) chlorine gas, control the feed time for 10h, and continue the reaction for 2h. After the reaction is completed, wash the oi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com