Method for reducing hydration heat release of magnesium cement material
A magnesia cementitious material and heat release technology, applied in the field of building materials, can solve the problems of large heat release and restrictions on large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
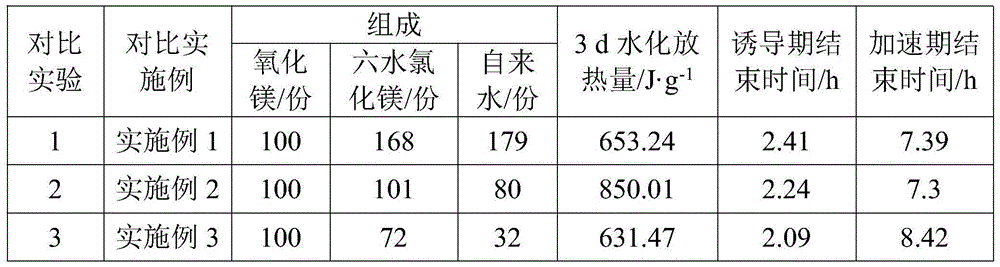
[0022] The raw material magnesia comes from the calcined product of magnesium slag, a by-product of lithium extraction from salt lakes, and the dosage is 129 parts. This example contains about 70% of the raw material magnesia for cement preparation, that is, about 90 parts of magnesia.
[0023] Dissolve 168 parts of bischofite (i.e. magnesium chloride hexahydrate) in 179 parts of tap water to form an aqueous solution of magnesium chloride, where the tap water is the added water, that is, the additional water added to the system, and the following refers to This meaning; then dissolve 129 parts of the above-mentioned calcined product and 10 parts of slag in the magnesium chloride aqueous solution, and stir evenly to form a magnesia gelling material, and the above-mentioned parts are parts by mass; that is to say, in the magnesia gelling material Among them, the mass ratio of ore mixed with magnesium oxide, magnesium chloride and water is 1.2:1:3.2, where water refers to the tota...
Embodiment 2
[0027] The raw material magnesium oxide is derived from dolomite lightly burned powder (the content of magnesium oxide is about 20% to 28%), that is, the calcined product of dolomite, and the consumption is about 388 parts. Among them, the content of magnesium oxide in this embodiment is 23% to 28%. 26%, that is to keep the consumption of raw material magnesia about 95 parts.
[0028] Dissolve about 101 parts of magnesium chloride hexahydrate in about 80 parts of tap water to form an aqueous magnesium chloride solution; then add about 388 parts of the above-mentioned dolomite lightly burned powder containing 23% to 26% of magnesium oxide and 5 parts of silica fume, and mix After being uniform, a magnesia gelling material is formed, and the above-mentioned parts are parts by mass; that is to say, in the magnesia gelling material, the mass ratio of magnesium oxide, magnesium chloride and water is 2.1:1:2.8, where water The source is the same as described in Example 1; and the ra...
Embodiment 3
[0031] The raw material magnesium oxide is derived from lightly burned magnesite powder, i.e. the calcined product of magnesite, with an amount of 115 parts, wherein the magnesium oxide content is 60% to 62%, that is to say, in the lightly burned magnesite powder of this embodiment The magnesium oxide content is about 70 parts.
[0032] Dissolve about 72 parts of magnesium chloride hexahydrate in about 32 parts of water to form a magnesium chloride aqueous solution, then dissolve 115 parts of the above-mentioned lightly burned magnesite powder and 30 parts of fly ash in the magnesium chloride aqueous solution, and stir evenly to form a magnesium gel material, the above-mentioned parts are all parts by mass; that is to say, in the magnesia gelling material, the mass ratio of ore-doped magnesium oxide, magnesium chloride and water is 2.8:1:2, and the source of water here is the same as in the embodiment 1; and the ratio of the amount of fly ash added to the mineral admixture to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydration enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com