Preparation method of 3-trifluoromethyl phenylacetonitrile
A technology of trifluoromethyl phenylacetonitrile and trifluoromethylation, which is applied in the field of preparation of m-trifluoromethyl phenylacetonitrile, can solve unsafe, clean production, low reaction yield, a large amount of waste sulfuric acid, fluorine-containing side effects Products and other issues, to achieve the effect of high yield, high product content, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
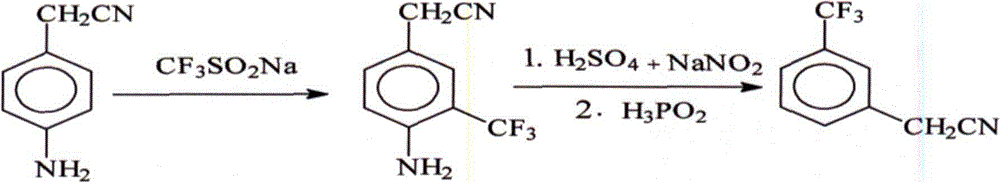
[0020] 33 grams (0.25 moles) of p-aminophenylacetonitrile, 45 grams (0.5 moles) of tert-butyl hydroperoxide and 78 grams (0.5 moles) of sodium trifluoromethanesulfinate were added to the reaction flask at room temperature and stirred for 4 hours. Add 500 milliliters of water and 200 milliliters of ethyl acetate to the reaction product, stir and separate layers, and the oil layer is the ethyl acetate solution of the intermediate 3-trifluoromethyl-4-aminophenylacetonitrile. After concentrating the ethyl acetate solution of 3-trifluoromethyl-4-aminophenylacetonitrile solvent, add 100 ml of water and 62.5 g (0.625 moles) of 98% concentrated sulfuric acid, cool to 0°C, keep warm at -2-5°C and drop Add 129.4 g (0.375 mol) of 20% sodium nitrite aqueous solution to carry out diazotization reaction, and stir for 30 minutes after the dropwise addition, and the prepared diazonium salt mixture is used for the next reaction.
[0021] Add 66 g (0.5 moles) of 50% hypophosphorous acid dropwis...
Embodiment 2
[0023] 33 grams (0.25 moles) of p-aminophenylacetonitrile, 112.5 grams (1.25 moles) of tert-butyl hydroperoxide and 195 grams (1.25 moles) of sodium trifluoromethanesulfinate were added to the reaction flask at room temperature and stirred for 2.5 hours, Add 1500 milliliters of water and 200 milliliters of ethyl acetate to the reaction product, stir and separate layers, and the oil layer is the ethyl acetate solution of the intermediate 3-trifluoromethyl-4-aminophenylacetonitrile. After concentrating the ethyl acetate solution of 3-trifluoromethyl-4-aminophenylacetonitrile solvent, add 200 ml of water and 125 g (1.25 moles) of 98% concentrated sulfuric acid, cool to 0°C, keep warm at -2-5°C and drop Add 172.5 g (0.5 moles) of 20% sodium nitrite aqueous solution to carry out diazotization reaction, and stir for 30 minutes after the dropwise addition, and the prepared diazonium salt mixture is used for the next reaction.
[0024] Add 165 g (1.25 moles) of 50% hypophosphorous aci...
Embodiment 3
[0026] Add 33 grams (0.25 moles) of p-aminophenylacetonitrile, 22.5 grams (0.25 moles) of tert-butyl hydroperoxide and 39 grams (0.25 moles) of sodium trifluoromethanesulfinate into the reaction flask and stir at 55-60°C for reaction After 2 hours, 400 milliliters of water and 200 milliliters of ethyl acetate were added to the reaction product, and the layers were stirred and separated. The oil layer was the ethyl acetate solution of the intermediate 3-trifluoromethyl-4-aminophenylacetonitrile. Concentrate the ethyl acetate solution of 3-trifluoromethyl-4-aminophenylacetonitrile solvent, add 100 ml of water and 50 g (0.5 moles) of 98% concentrated sulfuric acid, cool to 0°C, keep warm at 10-20°C and add dropwise 86.25 g (0.25 mole) of 20% sodium nitrite aqueous solution was subjected to diazotization reaction, and the dropwise addition was completed and stirred for 30 minutes, and the prepared diazonium salt mixture was used for the next reaction.
[0027] Add 33 grams (0.25 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com