A kind of d-dimer and fdp composite quality control product and preparation method thereof
A dimer, quality control product technology, applied in the preparation of test samples, material inspection products, biological testing and other directions, can solve the problems of affecting the accuracy of quality control, inconvenient use, etc., and achieve low production cost, low cost, Good reconstitution uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
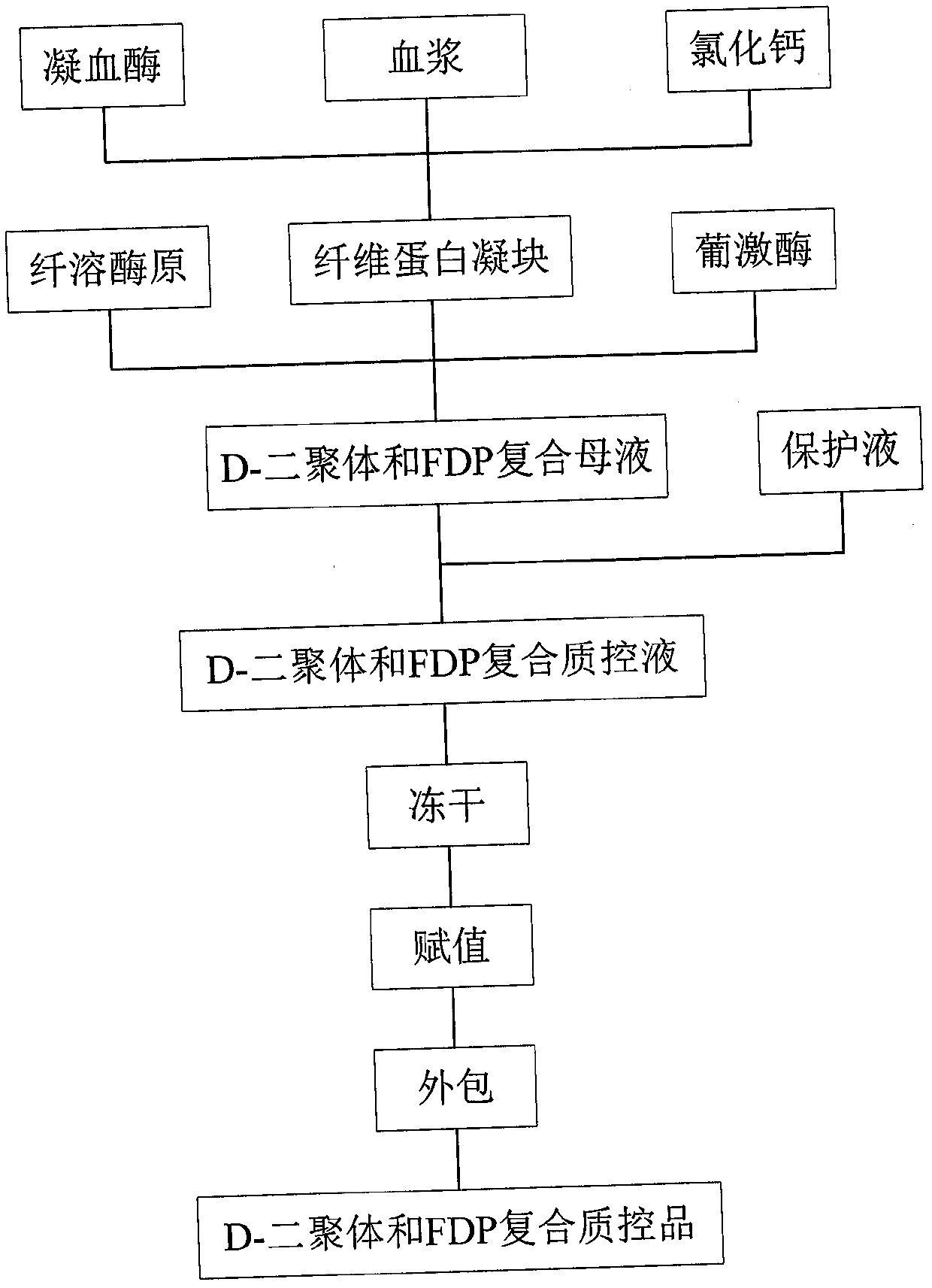
[0027] refer to figure 1 , a kind of preparation method of D-dimer and FDP composite quality control product, comprises the steps: 1) under the condition that calcium ion exists, add thrombin in blood plasma, make blood plasma coagulation process occur naturally at room temperature until complete coagulation ; At this time, the soluble fibrinogen in the plasma is completely converted into insoluble fibrin.
[0028] Plasma was purchased from human plasma as a commercial fibrinogen (FIB) quality control product or bovine plasma. A commercially available fibrinogen (FIB) quality control is preferably used. When bovine plasma is used, the bovine plasma comes from fresh blood from animal slaughterhouses, prepared by mixing blood with 0.109M trisodium citrate at a ratio of 9:1, centrifuging at 2000g-3000g for 30 minutes, and the upper layer is bovine plasma.
[0029] The calcium ion solution is 0.1mol / L-0.5mol / L calcium chloride reagent, and the added volume of the calcium ion sol...
Embodiment 1
[0033] Embodiment 1 A kind of preparation method of D-dimer and FDP composite quality control product
[0034] 1) Calcium chloride solution preparation: weigh 0.22g CaCl 2 .2H 2 0, add 5ml purified water, stir to make it dissolve, obtain the calcium chloride solution of 0.3mol / L.
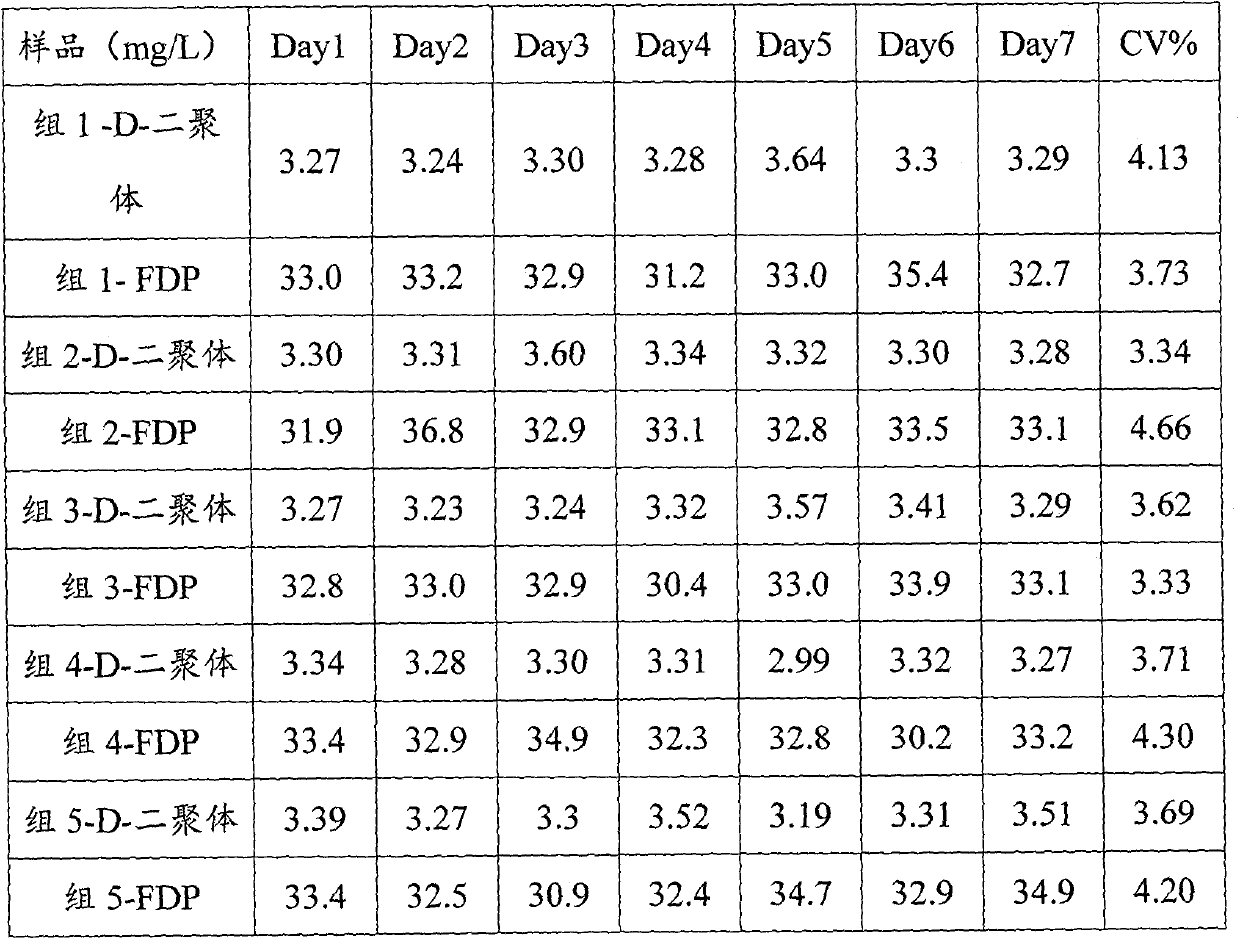
[0035] Fibrin clot preparation: Take 10 purchased FIB quality control products of 2 batches each, add 1ml of purified water to each tube and redissolve for 15 minutes, shake well in between to fully dissolve, group by batch number, respectively as group 1 (10ml), group 2 (10ml); take another 9ml of fresh bovine blood from 3 cows, mix with 1ml of 0.109M trisodium citrate respectively, centrifuge at 2000g for 30 minutes, carefully extract the supernatant, 3 cows The obtained bovine plasma was respectively recorded as group 3, group 4, and group 5. 1ml of 0.3mol / L calcium chloride solution and 500IU thrombin were added to the plasma of the 5 groups, shaken well, and left at room temperature for 4 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com