A kind of preparation method of d-dimer and fdp composite quality control product
A technique for dimers and quality control products, which is applied in the direction of analytical materials and instruments, can solve the problems of incomplete degradation of fibrin, lower quality of composite quality control products, and inactivation of fibrin, so as to avoid quality degradation and errors Small, high-purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
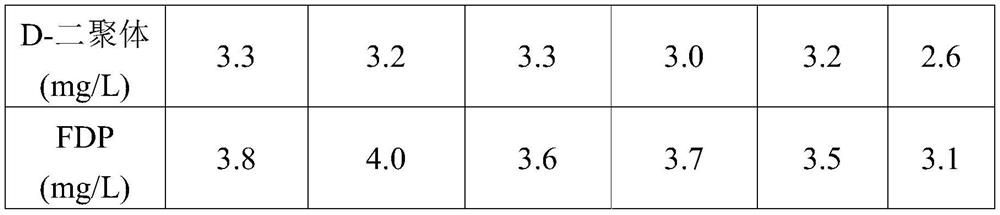
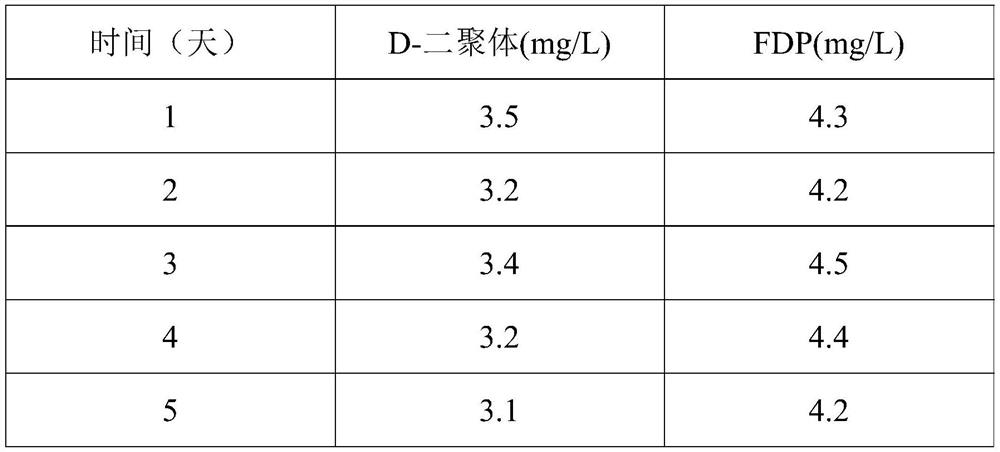
[0056] The preparation method of D-dimer and FDP composite quality control product in the present embodiment is as follows:
[0057] (1) In the SPF experiment, 150 mL of blood was collected from the rabbit heart and poured into a 250 mL Erlenmeyer flask with 30 glass beads with a diameter of 0.5 cm in advance, and shaken horizontally.
[0058] (2) The white fibrinogen floating on the blood obtained in step (1) was redissolved with sterile physiological saline.
[0059] (3) Use neutral protease with a concentration of 2000IU / mL to enzymolyze the fibrinogen obtained in step (2), relative to 1 mL of the fibrinogen solution reconstituted in step (2), the amount of protease in step (3) 50IU, 38°C for 10 hours, and then heated to 100°C for 2 hours to stop the enzymatic hydrolysis reaction, to obtain the D-dimer and FDP composite mother solution;
[0060] (4) The composite mother liquor obtained in step (3) is diluted with a protective solution consisting of HEPES 2% (w / v), trehalos...
Embodiment 2
[0062] The preparation method of D-dimer and FDP composite quality control product in the present embodiment is as follows:
[0063] (1) In the SPF experiment, 200 mL of blood was taken from the bovine heart and poured into a 500 mL Erlenmeyer flask pre-placed with 35 glass beads with a diameter of 0.7 cm, and shaken horizontally.
[0064] (2) The white fibrinogen floating on the blood obtained in step (1) was redissolved with sterile physiological saline.
[0065] (3) Use neutral protease with a concentration of 2000IU / mL to enzymolyze the fibrinogen obtained in step (2), relative to 1 mL of the fibrinogen solution reconstituted in step (2), the amount of protease in step (3) 80IU at 35°C for 15 hours, then raised to 50°C for 8 hours to stop the enzymatic hydrolysis reaction, to obtain D-dimer and FDP composite mother solution.
[0066] (4) The composite mother liquor obtained in step (3) is diluted with a protective solution consisting of HEPES 1% (w / v), trehalose 1g / L, bov...
Embodiment 3
[0068] The preparation method of D-dimer and FDP composite quality control product in the present embodiment is as follows:
[0069] (1) Take 120 mL of blood from the heart of the SPF experimental rat and pour it into a 150 mL Erlenmeyer flask with 25 glass beads with a diameter of 0.3 cm in advance, and shake it horizontally.
[0070] (2) The white fibrinogen floating on the blood obtained in step (1) was redissolved with sterile physiological saline.
[0071] (3) Use neutral protease with a concentration of 2000IU / mL to enzymolyze the fibrinogen obtained in step (2), relative to 1 mL of the fibrinogen solution reconstituted in step (2), the amount of protease in step (3) 100IU, 36°C enzymatic hydrolysis for 13 hours, then raised to 70°C for 5 hours to terminate the enzymatic hydrolysis reaction, to obtain D-dimer and FDP composite mother solution;
[0072] (4) The composite mother liquor obtained in step (3) is diluted with a protective solution consisting of HEPES 3% (w / v)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com