Preparation method of 2-chloro nicotinaldehyde
A technology of nicotinic aldehyde and chloronicotinic acid, applied in the direction of organic chemistry and other directions, can solve the problems of blank in industrial synthesis and large-scale production, no value of industrial production, expensive reaction reagents, etc., and achieves low cost of raw materials, mild reaction conditions, and ease of use. The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
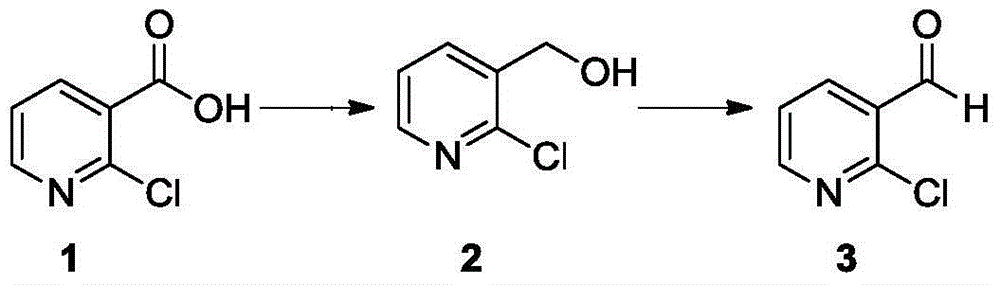
[0015] (1) Synthesis of 2-chloronicotinol
[0016] Add NaBH to the four-neck flask 4 (72g) and tetrahydrofuran 450ml, cooled to -10℃~0℃. Boron trifluoride diethyl ether solution (575g) was added dropwise, and the internal temperature was controlled to be <10°C. After dropping, stirred for 5 minutes. Then add 2-chloronicotinic acid (100g) and tetrahydrofuran 600ml mixed solution dropwise, control the temperature <30°C, after dropping, raise the temperature to room temperature and react for 6h. Adjust the pH to 8-9, filter with suction, extract the filtrate twice with ethyl acetate, dry the organic layer with anhydrous sodium sulfate, filter with suction, and concentrate to obtain 125 g of the product, which is directly used in the next reaction.
[0017] (2) Synthesis of 2-chloronicotinaldehyde
[0018] Add 2-chloronicotinol (125g) and 450ml of dichloromethane into the four-necked bottle, stir evenly, add manganese dioxide (280g), heat up to reflux for 3 hours, after the rea...
Embodiment 2
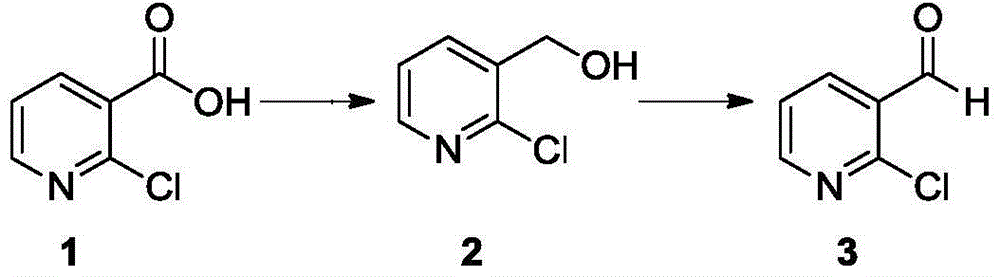
[0020] (1) Synthesis of 2-chloronicotinol
[0021] Add NaBH to the four-neck flask 4 (80g) and 500ml of tetrahydrofuran, cooled to -10°C to 0°C. Boron trifluoride diethyl ether solution (590g) was added dropwise, and the internal temperature was controlled to be <10°C. After dropping, stirred for 5 minutes. Then add 2-chloronicotinic acid (100g) and tetrahydrofuran 550ml mixed solution dropwise, control the temperature <25°C, after dropping, raise the temperature to room temperature and react for 8h. Adjust the pH to 8-9, filter with suction, extract the filtrate twice with ethyl acetate, dry the organic layer with anhydrous sodium sulfate, filter with suction, and concentrate to obtain 131 g of the product, which is directly used in the next reaction.
[0022] (2) Synthesis of 2-chloronicotinaldehyde
[0023] Add 2-chloronicotinol (131g) and 460ml of dichloromethane into the four-necked bottle, stir well, add manganese dioxide (300g), heat up to reflux reaction for 5h, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com