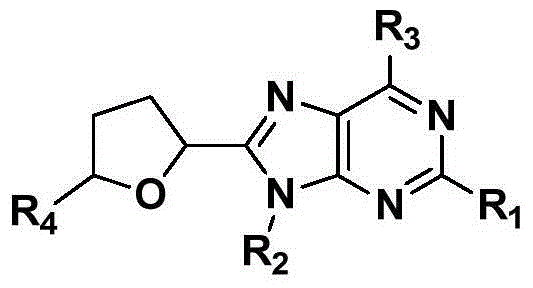
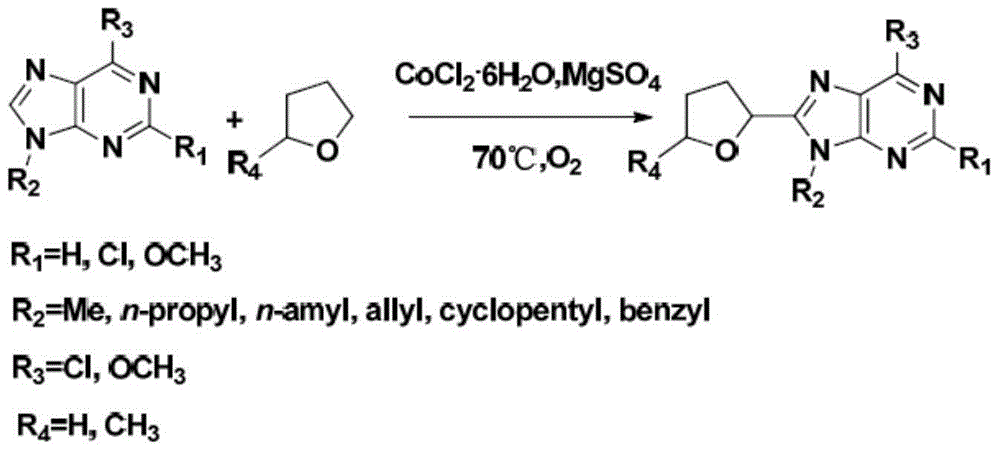
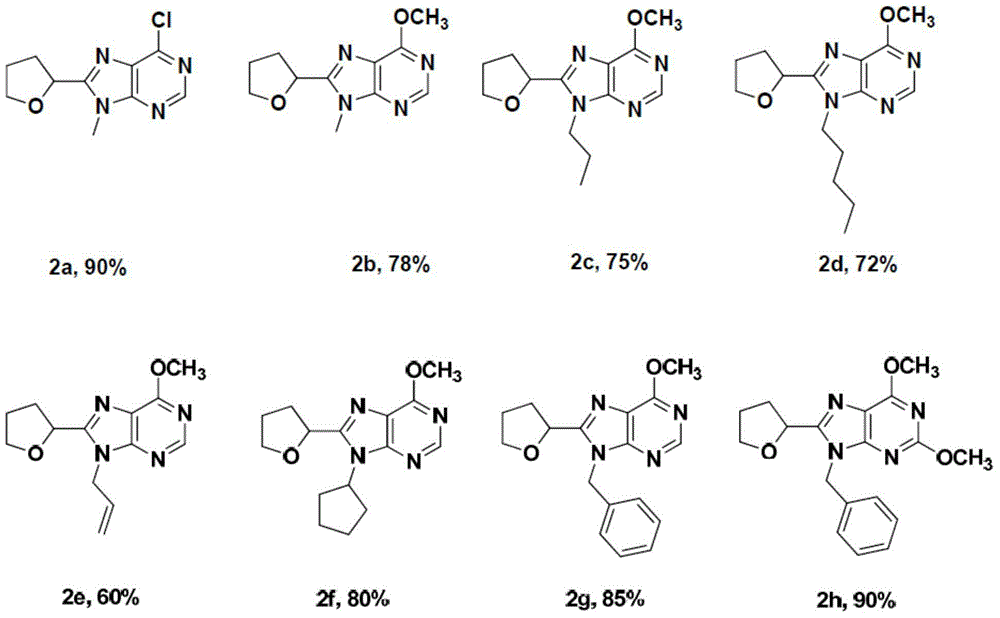
Novel C8-heterocyclic alkylated purine nucleoside analogue and synthetic method thereof
A technology for heterocyclic alkylation of purine nucleosides and a synthetic method, which is applied in the fields of chemistry and medicine, and can solve the problems of expensive raw materials, complex processes, and harsh reaction conditions, and achieve the effects of easy access, avoiding reaction conditions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 6-methoxy-9-benzylpurine (0.125mmol, 0.0301g) into a 25ml reaction tube, CoCl 2 ·6H 2 O (20mol%, 0.00593g), Mg 2 SO 4 (0.75mmol, 0.075g) and 2mL THF (both raw material and solvent). The reaction mixture was reacted at 50°C for 72 hours under the protection of oxygen. Track the reaction with TLC. After terminating the reaction, the reaction solution was cooled to room temperature, and the solvent was evaporated to dryness by a rotary evaporator. The resulting residue was purified by column chromatography to obtain the target compound 6-methoxy-8-(2-tetrahydrofuran)-9 -Benzyl purine, yield 85%.
Embodiment 2
[0034] Add 6-methoxy-9-benzylpurine (1.25mmol, 0.301g) to a 100ml reaction tube, CoCl 2 ·6H 2 O (20mol%, 0.0593g), Mg 2 SO 4 (7.5mmol, 0.75g) and 20mL THF (both raw material and solvent). The reaction mixture was placed in an oil bath at 70° C. and stirred for 48 hours under the protection of oxygen. Track the reaction with TLC. After terminating the reaction, the reaction solution was cooled to room temperature, and the solvent was evaporated to dryness by a rotary evaporator. The resulting residue was purified by column chromatography to obtain the target compound 6-methoxy-8-(2-tetrahydrofuran)-9 -Benzyl purine, yield 80%.
Embodiment 3
[0036] Add 6-methoxy-9-benzylpurine (12.5mmol, 3.01g) in a 500ml reaction tube, CoCl 2 ·6H 2 O (20mol%, 0.593g), Mg 2 SO 4 (75mmol, 7.5g) and 200mL THF (both raw material and solvent). The reaction mixture was stirred in an oil bath at 90°C for 48 hours under the protection of oxygen. Track the reaction with TLC. After terminating the reaction, the reaction solution was cooled to room temperature, and the solvent was evaporated to dryness by a rotary evaporator. The resulting residue was purified by column chromatography to obtain the target compound 6-methoxy-8-(2-tetrahydrofuran)-9 -Benzyl purine, yield 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com