Primers, probes and kits for quantitative detection of spinal muscular atrophy genes
A spinal muscular atrophy, fluorescent quantitative detection technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of long operation time, few detection sites, non-specificity, etc. To achieve the effect of avoiding severe children, preventing false negatives, and shortening the operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1. Technical basis
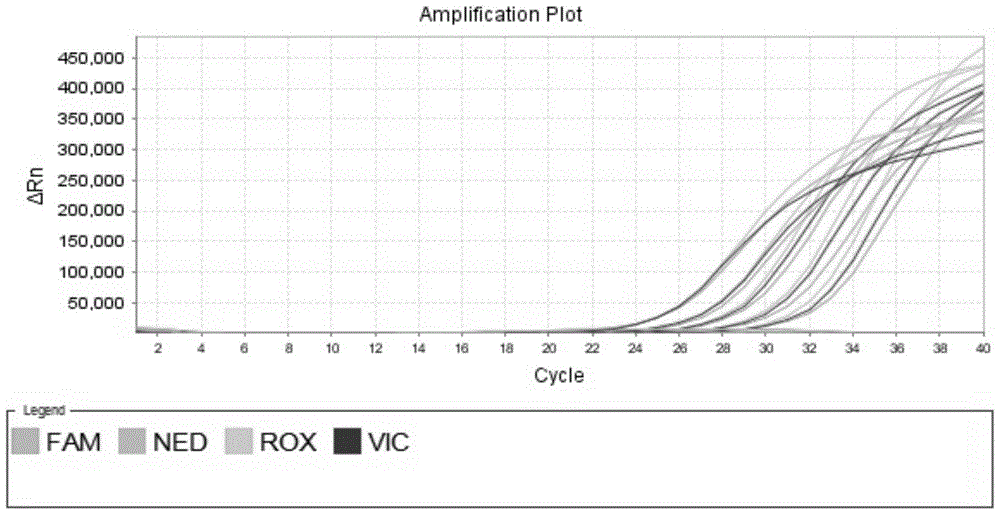
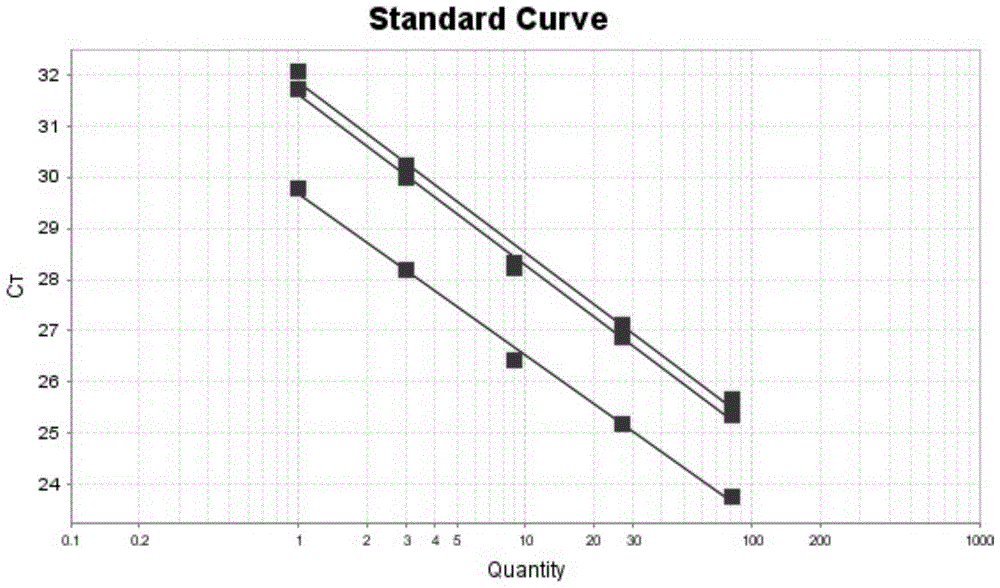
[0076] The present invention adopts Taq Man-MGB probe technique combined with relative quantitative method to analyze. The 5′ end of the Taq Man probe is labeled with a reporter group (Reporter, R), such as FAM, VIC, NED, etc., and the 3′ end is labeled with a fluorescent quencher group (Quencher, Q), such as TAMRA, etc. When the probe When intact, both can undergo fluorescence resonance energy transfer (FRET), and no fluorescent signal can be detected. During PCR amplification, the 5′ exonuclease activity of Taq enzyme hydrolyzes the probe, increasing the distance between the fluorescent molecule and the quencher molecule, thereby destroying its FRET, and the fluorescence monitoring system can detect the fluorescent signal. The quenching group of the MGB probe adopts a non-fluorescent quenching group (Non-Fluorescent Quencher), which does not generate fluorescence itself, and can greatly reduce the intensity of the background signal. At the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com