Quality control method for Huang's sound pill
A technology of Huang's Xiangsheng pill and detection method, which is applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of quality inspection interference, inaccurate test results, and inability to accurately and comprehensively control product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
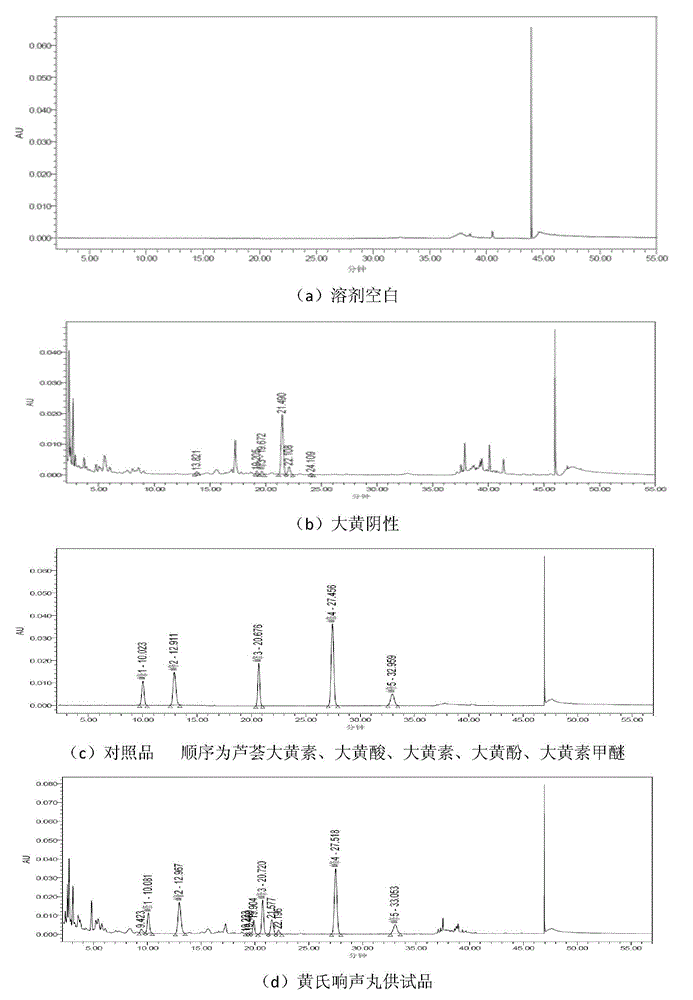
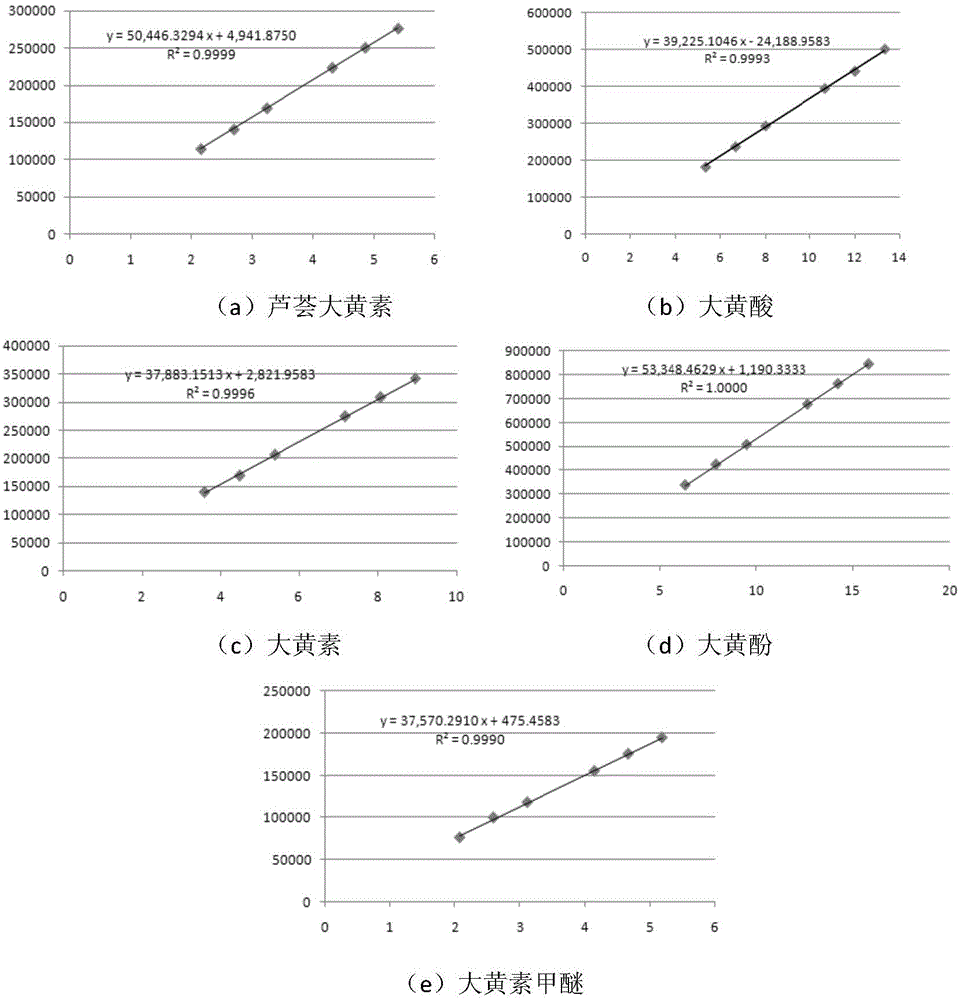
[0075] The content assay method of embodiment 1 free anthraquinone and combined anthraquinone
[0076] 1. Chromatographic conditions:
[0077] Chromatographic instrument: Waters H-class UPLC, chromatographic column: Phonomenex Gemini-NX C18 (150×4.6mm, 5um), column temperature: 30°C, flow rate: 1.0ml / min, mobile phase: acetonitrile-0.32% phosphoric acid aqueous solution 38: 62 hold for 15 minutes, adjust to 51:49, hold for 8 minutes, adjust to 55:45, hold for 12 minutes, detection wavelength: 254nm, injection volume: 10ul.
[0078] 2. Sample preparation
[0079] Preparation of the reference substance solution: Take an appropriate amount of the aloe-emodin reference substance and accurately weigh it, add methanol to make a reference substance solution containing 0.03 mg per 1 ml. Take an appropriate amount of emodin reference substance and accurately weigh it, add methanol to make a reference solution containing 0.05 mg per 1 ml. Take an appropriate amount of rhein reference...
Embodiment 2
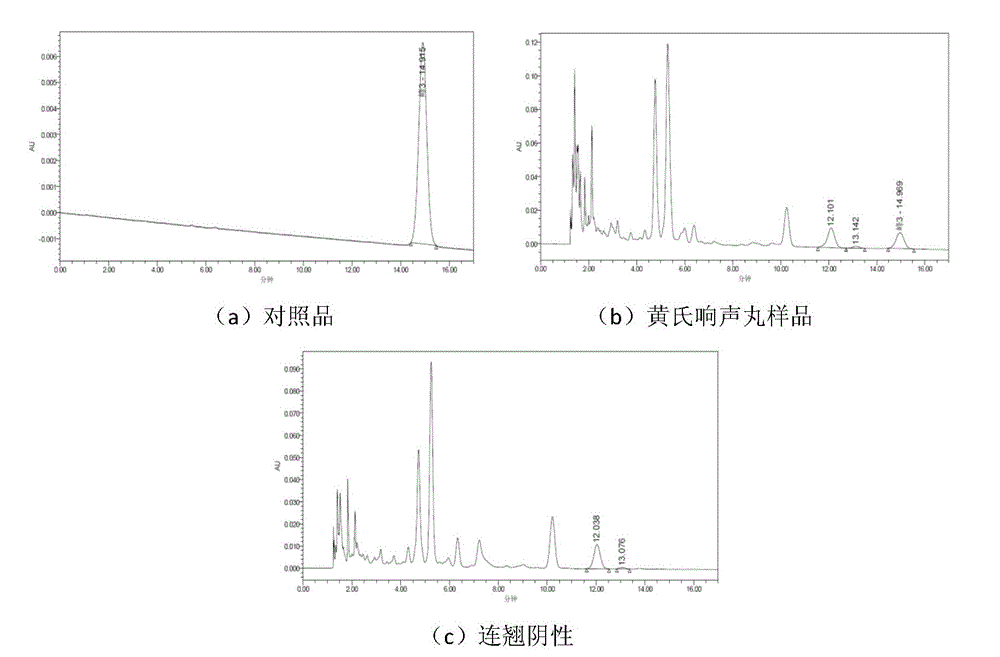
[0105] The content assay method of embodiment 2 forsythin
[0106] 1. Chromatographic conditions: Chromatographic instrument: Waters H-class UPLC, chromatographic column: Phonomenex Gemini-NX C18 (150×4.6mm, 5um), column temperature: 40°C, flow rate: 1.0ml / min, mobile phase: acetonitrile-water (21:79), detection wavelength: 277nm, injection volume: 10ul
[0107] 2. Sample preparation
[0108] Preparation of the reference substance solution: Accurately weigh 5.64 mg of the reference substance forsythin, place it in a 25ml volumetric flask, dissolve the mobile phase, and constant volume. The reference substance stock solution (0.2150 mg / ml) was obtained.
[0109] Preparation of the test solution: Take 1g of Huangshi Xiangsheng pills, accurately weighed, put in a stoppered Erlenmeyer flask, add 20ml of methanol, seal it tightly, ultrasonicate for 30min, accurately measure 10ml of the subsequent filtrate, steam to nearly dry, add medium Mix 1.5 g of neutral alumina (100-200 mes...
Embodiment 3
[0133] The assay method of embodiment 3 ferulic acid
[0134] 1. Chromatographic conditions: chromatograph: Waters H-class UPLC, chromatographic column: Phonomenex Gemini-NX C18 (150×4.6mm, 5um), column temperature: 35°C, flow rate: 1.0ml / min, mobile phase: methanol-0.1 % phosphoric acid solution (20:80), detection wavelength: 321nm, injection volume: 10ul
[0135] 2. Sample preparation
[0136] Preparation of the reference substance solution: Take an appropriate amount of the ferulic acid reference substance and accurately weigh it, add 70% methanol to make a reference substance solution containing 0.05 mg per 1 ml.
[0137] Preparation of the test solution: Take 1g of Huangshi Xiangsheng Pills, accurately weighed, add 20ml of 70% methanol solution, ultrasonic treatment for 10min, filter, and get the filtrate.
[0138] Determination: Precisely pipette the reference substance solution and the test solution of the same volume for sample injection, the volume drawn is 10ul, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com