Polymer coatings
A polymer coating technology, applied in the fields of chemistry, biology and material science, can solve the problem of inconvenient coating beads
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0257] In some embodiments of the above-described methods of making a polymeric coating affixed to a substrate surface, said polymeric coating is dissolved in an aqueous solution prior to covalently binding to said functional groups of said surface. In some embodiments, the matrix is a bead.
[0258] A second aspect of the methods disclosed herein relates to methods for preparing polynucleotide arrays. In such an embodiment, the method may comprise the step of: (a) coating a plurality of first oligonucleotides and a plurality of second oligonucleotides with a polymer coating present on the surface of any one of the substrates described herein. Reactive sites on a polymer coating prepared by or by any of the methods described herein for immobilizing a polymer coating to a substrate surface; (b) said plurality of contacting a first oligonucleotide with a template to be amplified; and (c) amplifying the template using the first oligonucleotide and the second oligonucleotide, the...
Embodiment 1
[0303] Preparation of PAZAM
[0304] overview
[0305] Unless otherwise stated, all reactions were performed under nitrogen or argon atmosphere and starting materials were obtained from suppliers (Aldrich Chemical Company, Fisher Scientific, Dow) and used as received without further purification. All reaction temperatures reported indicate the temperature of the bath / air in contact with the reaction vessel. Anhydrous solvents are not required.
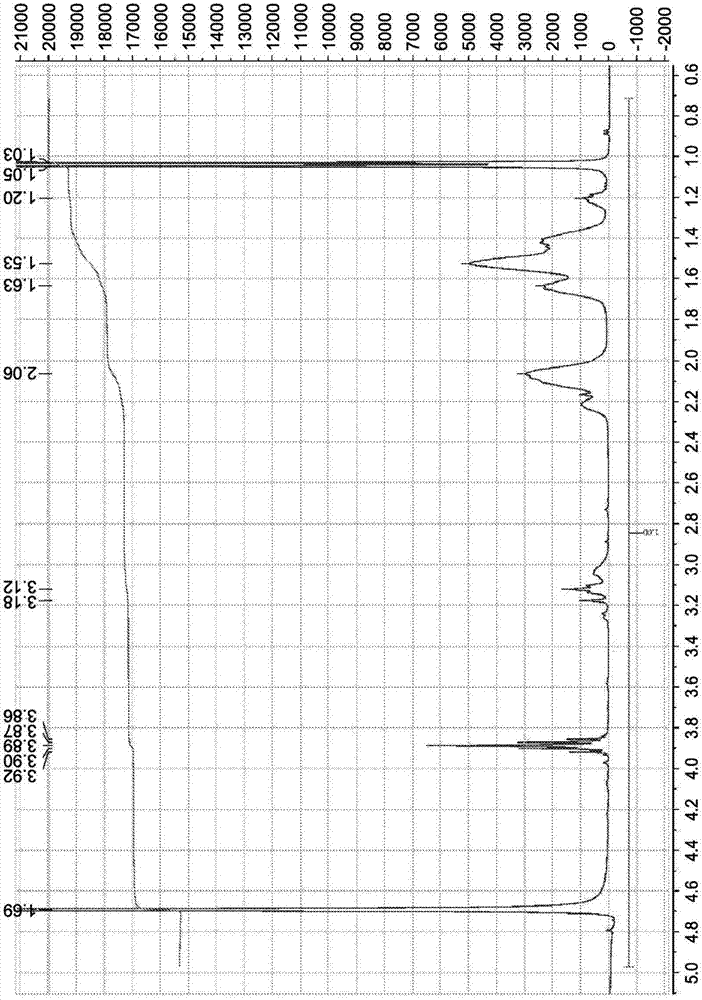
[0306] Recorded as deuterium oxide (99.9 at. %D for LC NMR) on a Bruker Avance 400 MHz instrument 1 H-NMR spectrum and 13 C-NMR spectrum. Chemical shifts are expressed in parts per million (ppm, δ) downfield from tetramethylsilane (TMS) and are referenced to the indicated solvent as internal standard.
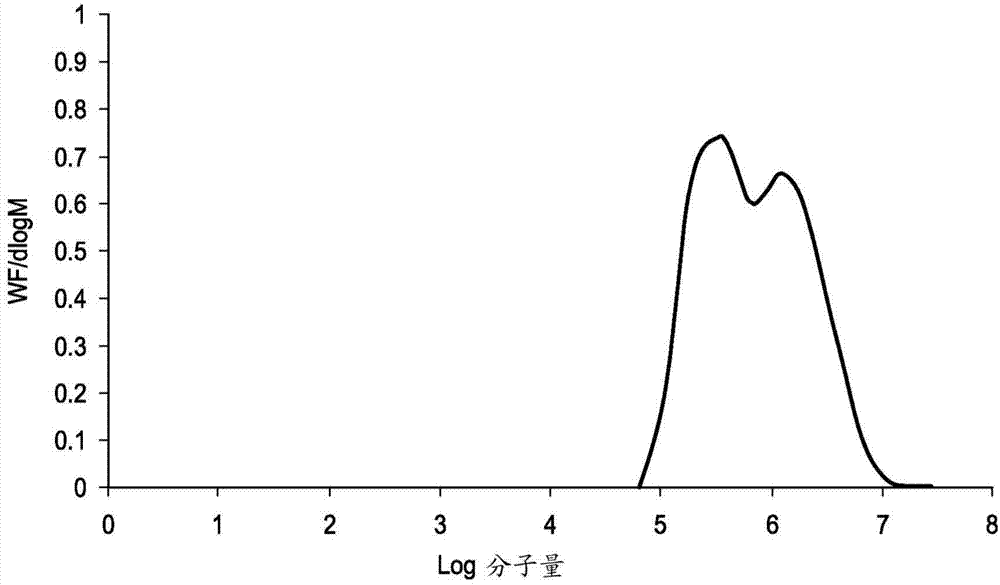
[0307] GPC analysis was performed by Smithers Rapra Technology Limited using the following chromatographic conditions:
[0308] Instrumentation: Malvern / Viscotek Triple Detector Array TDA301, combined pump and autosampler.
...
Embodiment 2
[0347] Preparation of PAZAM derivatives
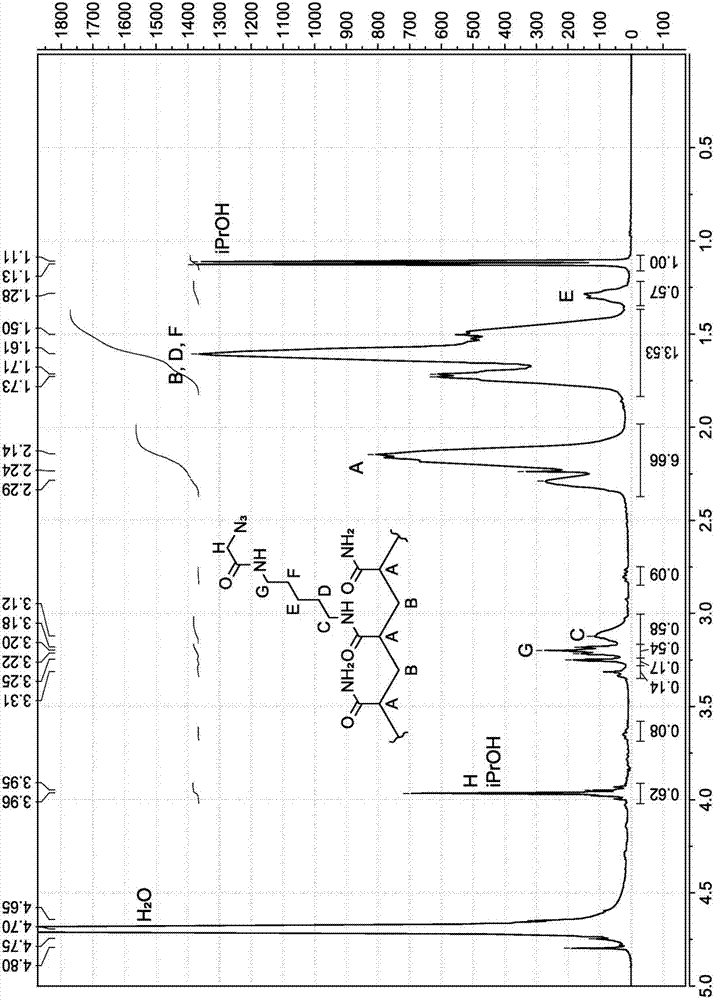
[0348] The synthesis of PAZAM derivatives is shown in Scheme 2. First, BRAPA (1c) was reacted with t-Boc protected hydroxylamine (2a) to form intermediate (2b), which was treated with dichloroacetic acid to form the oxoamine derivative of PAZAM (2c). 2c can then be grafted with aldehyde functionalized oligonucleotides to form 2d.
[0349] Scheme 2. Synthesis of oxoamine derivatives of PAZAM
[0350]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com