A kind of desulfurization catalyst and its preparation method and the method of hydrocarbon oil desulfurization
A desulfurization catalyst, oxide technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of reducing the activity of adsorbents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
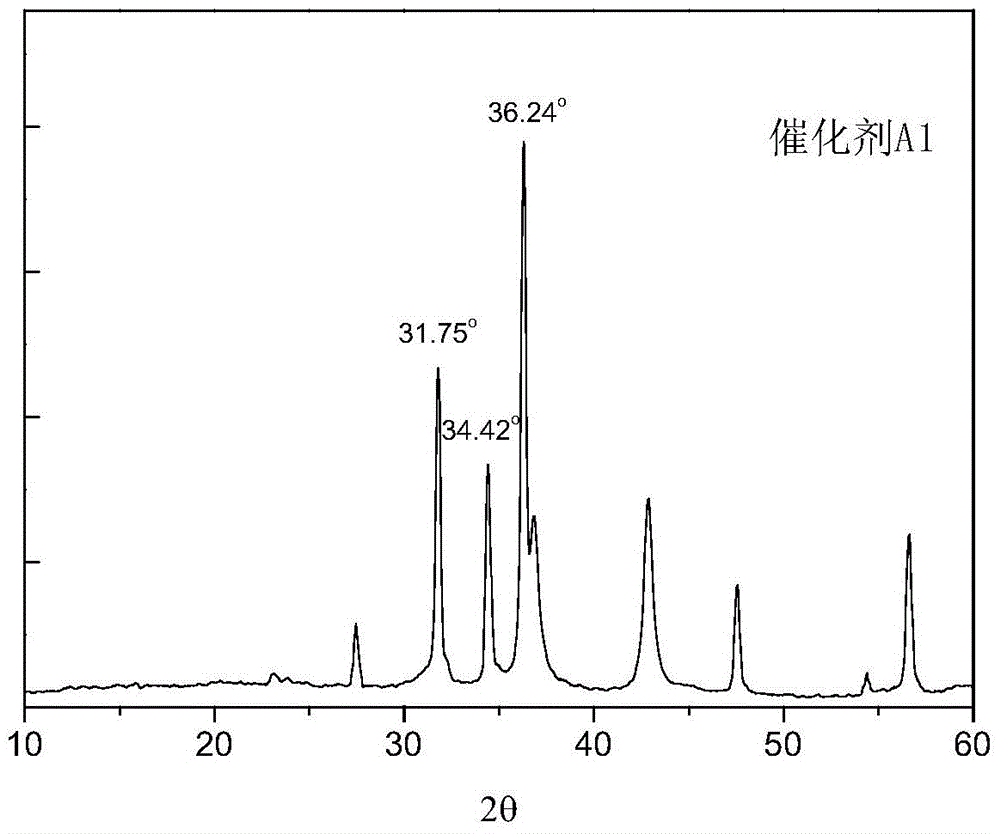
Image
Examples
preparation example Construction
[0040] The present invention also provides a method for preparing a desulfurization catalyst. The method includes: (1) subjecting a mixed solution obtained by mixing a lead-containing compound, a zinc-containing compound and water to a precipitation reaction, and filtering, drying, and filtering the mixture obtained by the precipitation reaction. Roasting to obtain a precipitated product; (2) contacting a silicon oxide source, a non-aluminum oxide precursor, water and an acid solution to form a slurry, and mixing the precipitated product obtained in step (1) with the slurry to form a carrier mixture; The carrier mixture is shaped, dried and calcined to form a carrier; (3) a compound containing an active metal is introduced into the carrier obtained in step (2), dried and calcined to obtain a desulfurization catalyst precursor; the active metal is cobalt, At least one of nickel, iron and manganese; (4) reducing the desulfurization catalyst precursor obtained in step (3) in an atm...
Embodiment 1
[0081] This example is used to illustrate the preparation method of the desulfurization catalyst of the present invention.
[0082] (1) Prepare precipitation product. Mix 10.9 kilograms of zinc acetate dihydrate powder (produced by Beijing Chemical Plant, analytically pure), 0.86 kilograms of lead acetate trihydrate (Sinopharm Chemical Reagent Company, analytically pure), and 18 kilograms of deionized water, stirred for 30 minutes and then completely dissolved. The precipitated product obtained by adding 1.8 kg of ammonia water was filtered and dried at 150°C for 2 hours, and then calcined at 500°C for 1 hour to obtain the precipitated product C1.
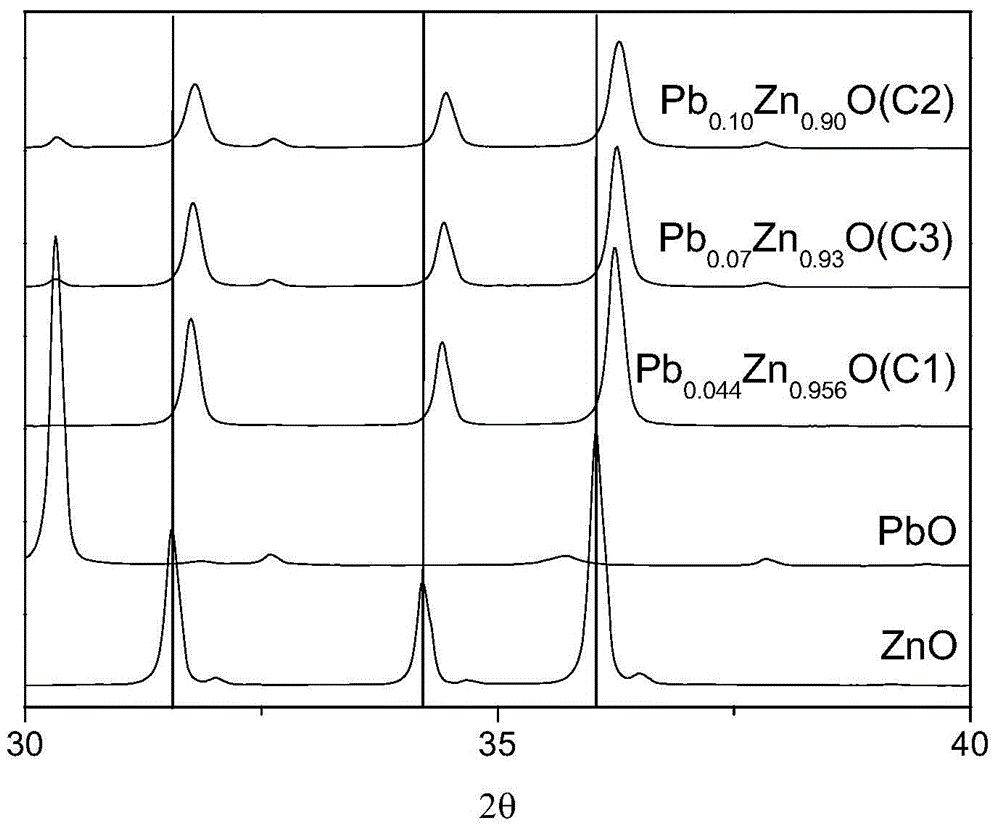
[0083] The precipitated product C1 was subjected to fluorescence analysis and XRD measurement. XRD spectrum (see figure 1 ) There is no diffraction peak of PbO, and the standard diffraction peak of ZnO shifts to the right, where A 100 -B 100 =0.2°, A 002 -B 002 = 0.21°, A 101 -B 101 =0.2°, indicating that PbO and ZnO in the precipitati...
Embodiment 2
[0092] This example is used to illustrate the preparation method of the desulfurization catalyst of the present invention.
[0093] (1) Prepare precipitation product. Mix 14.0 kg of zinc nitrate hexahydrate (produced by Beijing Chemical Plant, analytically pure), 1.80 kg of lead nitrate powder (Sinopharm Chemical Reagent Company, analytically pure), and 18 kg of deionized water. After stirring for 30 minutes, add 2.0 kg of urea and heat to A white precipitate was obtained after treatment at 80°C for 2 hours. After filtration, it was dried at 150°C for 2 hours, and then calcined at 500°C for 1 hour to obtain precipitated product C2.
[0094] The precipitation product C2 was subjected to fluorescence analysis and XRD measurement. XRD spectrum (see figure 1 ) There is no diffraction peak of PbO, and the standard diffraction peak of ZnO shifts to the right, where A 100 -B 100 =0.25°, A 002 -B 002 = 0.24°, A 101 -B 101 = 0.24°, indicating that PbO and ZnO in the precipitation product C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com