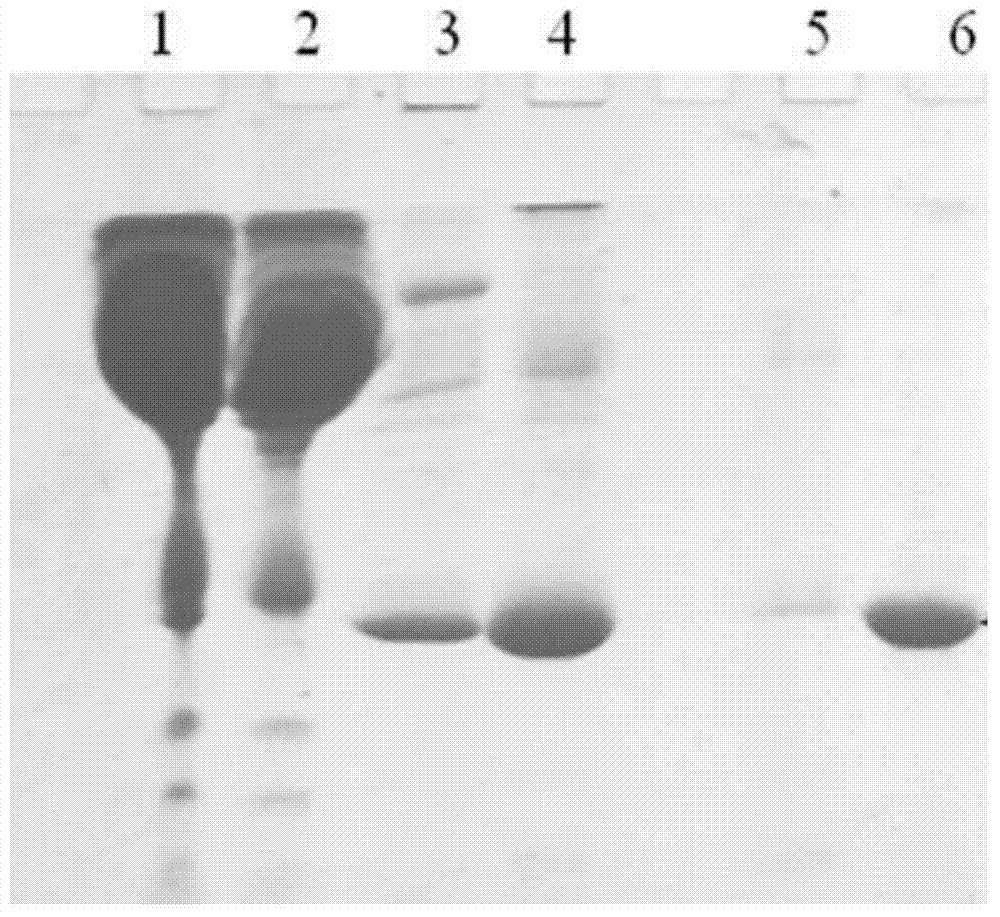
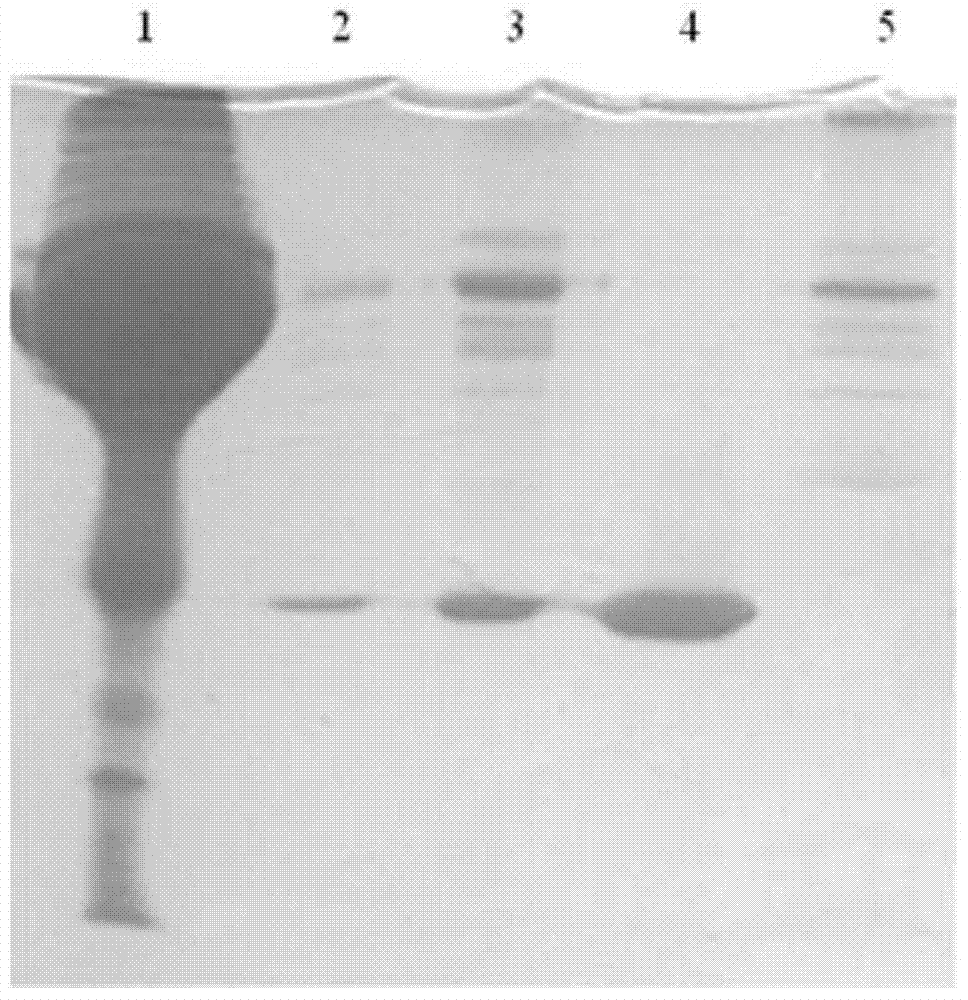
Apolipoprotein A1 purification method and ApoAI protein injection antigen
An apolipoprotein and purification method technology, applied in the field of biological separation, can solve the problems of limited sample volume, reduced detection accuracy, expensive processing equipment, etc., and achieves the effects of simple and novel method, low cost, and large amount of processed samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In this example 1, the hydrophobic chromatography packing material phenyl sepharose high performance was used to treat the static human plasma.
[0050] S11, equilibrate the chromatographic column filled with phenyl sepharose high performance filler, with a height of 10cm and a diameter of 1.6cm, with equilibrium solution A (25mM Tris-HCl+25mM NaCl at pH 7.4), and equilibrate to the liquid outlet of the chromatographic column The pH value is the same as that of the balance solution;
[0051] S12, after the static human plasma is centrifuged at a low speed (4000g), the clear liquid after removing suspended particles is used as a sample for later use.
[0052] S21, after diluting 20ml of the sample in step S12 with plasma diluent (25mM Tris-HCl pH7.4) to 4 times the volume, directly load the sample on the above-mentioned chromatographic column equilibrated with equilibrium solution A. After the sample loading is completed, use the balance solution A to wash the unbound h...
Embodiment 2
[0060] S11, equilibrate the chromatographic column filled with phenyl sepharose high performance filler, with a height of 10cm and a diameter of 1.6cm, with equilibrium solution A (25mM Tris-HCl+25mM NaCl at pH 7.4), and equilibrate to the liquid outlet of the chromatographic column The pH value is the same as that of the balance solution;
[0061] S12, after the static human plasma is centrifuged at a low speed (4100g), the clear liquid after removing suspended particles is used as a sample for later use.
[0062] S21, after diluting 20ml of the sample in step S12 with plasma diluent (25mM Tris-HCl pH7.4) to 4 times the volume, directly load the sample on the above-mentioned chromatographic column equilibrated with equilibrium solution A. After the sample loading is completed, use the balance solution A to wash the unbound human plasma components in the chromatography filler; wherein, the control flow rate during the balance, sample loading and washing processes is 3ml / min; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com