A human monoclonal antibody against the vp1 protein of jc virus
A monoclonal antibody, JC virus technology, applied in the direction of antiviral immunoglobulins, antibodies, antiviral agents, etc., can solve the problem of not raising or mentioning neutralizing antibodies in any way
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] By methods similar to those previously described (Plaisant, P., et al., Human monoclonal recombinant Fabs specific for HCV antigens obtained by repertoire cloning in phage display combinatorial vectors. Res Virol, 1997.148(2): p.165-9 ) was constructed in pPD phagemid vector of IgG1 / k isotype and had 2x10 7 A combinatorial phage-display library of human antibody fragments (monovalent Fabs) at an estimated scale of three elements. The library was generated from the bone marrow of a 68-year-old male whose serum tested positive for the presence of anti-VP1 / JCV antibodies by ELISA. By using 100ng / well of recombinant JCV VP1 protein (Mad1, ) to coat a 96-well plate for ELISA. Several serum dilutions were added to VP1 coated plates in duplicate. The plate was incubated at 37°C for 1 hour and then washed with 0.1% PBS / TWEEN 20. Anti-human IgG1 antibody conjugated with peroxidase (HRP) ( ) to detect bound antibodies.
[0034] Carry out as previously described (Williamso...
Embodiment 2
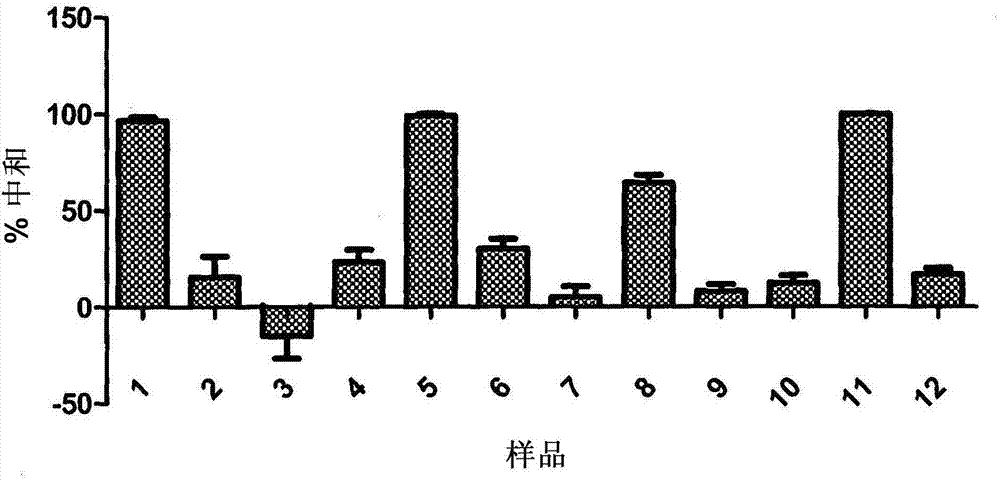
[0061] Defining the (linear or conformational) nature of the epitope recognized by the GRE1 monoclonal antibody
[0062] To define the (linear or conformational) nature of the epitope recognized by the GRE1 monoclonal antibody, Western Blot and Dot Blot were performed with both denatured and wild-type protein.
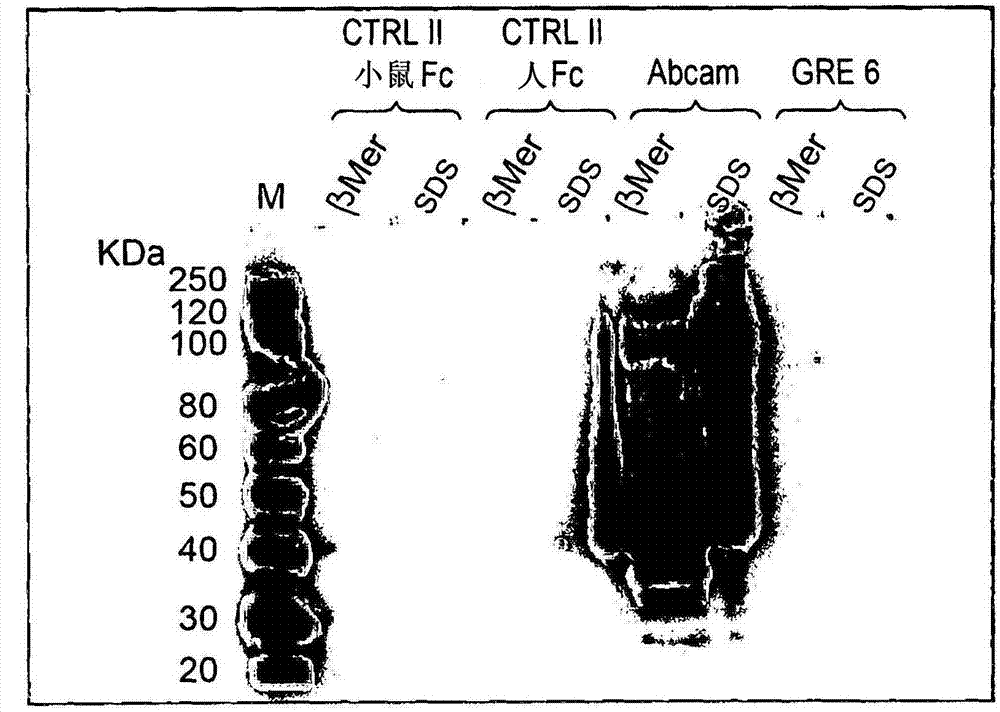
[0063] figure 2 Results of immunoblotting under denaturing conditions are shown. Proteins were denatured with β-mercaptoethanol (β-mer) or with sodium dodecyl sulfate (SDS). The commercial murine antibody was designated as Abcam, and the GRE1 anti-JCV VP1 monoclonal antibody was designated as GRE.
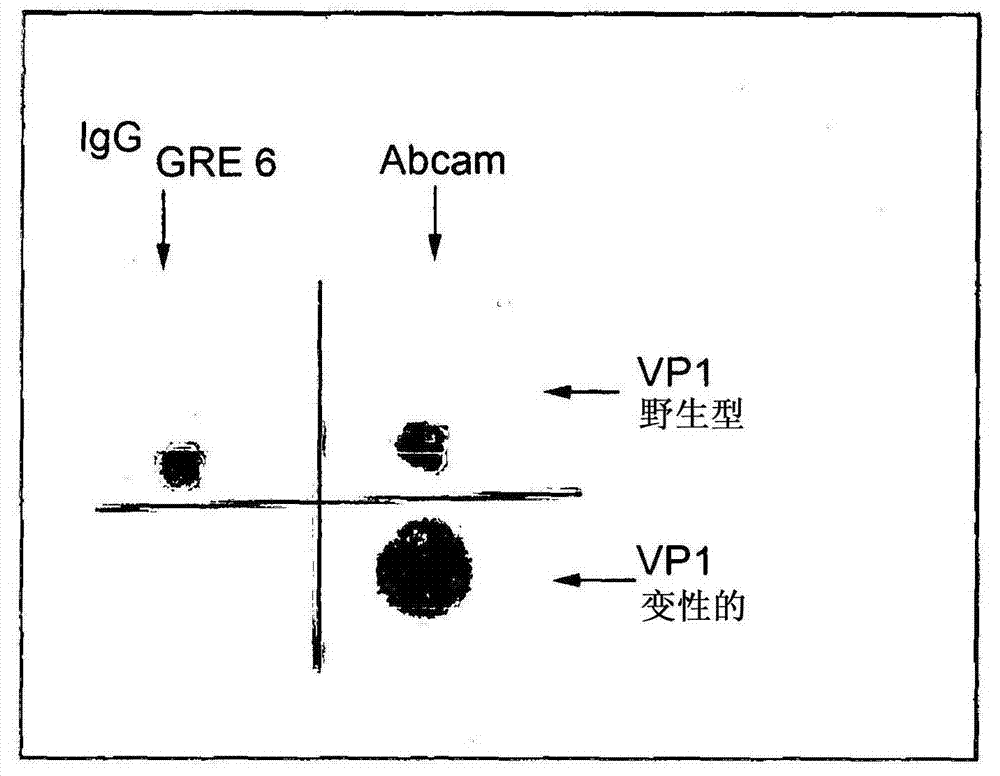
[0064] image 3 Results of dot blot experiments with both denatured VP1 and VP1 in wild-type conformation are shown. The commercial murine antibody was designated Abcam, while the GRE1 anti-JCV VP1 monoclonal antibody was designated IgG GRE.
[0065] The results showed that GRE1 was unable to bind to denatured forms of the protein, whereas it was only able to recogn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com