Topiroxostat dispersible tablets and preparation method of topiroxostat dispersible tablets
A technology of topinostat and dispersible tablets, which is applied in the field of pharmaceutical preparations, can solve problems such as unstable quality and low dissolution rate, and achieve the effects of good drug efficacy, high bioavailability, and fast absorption by the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
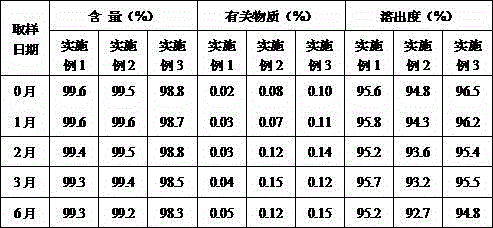
Examples
Embodiment 1
[0029] Prescription (specification: 20mg):
[0030] Topicastat 20.0 g
[0031] Microcrystalline cellulose 60.0 g
[0032] Lactose 20.0 g
[0033] Crospovidone 40.0 g
[0035] Meglumine 6.0 g
[0036] Povidone K30 2.0 g
[0037] Magnesium stearate 2.0 g
[0038] Micronized silica gel 2.0 g
[0039] A total of 1000 pieces were made
[0040] Preparation:
[0041] ① Treatment of raw and auxiliary materials: Dissolve sodium hydroxide and meglumine in 9 g of purified water, add 25 g of ethanol, and then add the prescribed amount of topinostat, and stir until completely dissolved; pass the rest of the auxiliary materials through a standard sieve above 60 mesh for later use.
[0042] ② Weigh the diluent, adhesive and 30g crospovidone according to the prescription and mix evenly, add the above liquid to make a soft material
[0043] ③ Wet granulation: take the above-mentioned soft material and pass it through a 24-mesh sieve to...
Embodiment 2
[0049] Prescription (specification: 40mg):
[0050] Topicastat 40.0 g
[0051] Microcrystalline Cellulose 100.0 g
[0052] Lactose 40.0 g
[0053] Sodium starch glycolate 80.0 g
[0055] Meglumine 15.0 g
[0056] Hypromellose 5.0 g
[0057] Magnesium stearate 4.0 g
[0058] Micronized silica gel 4.0 g
[0059]A total of 1000 pieces were made
[0060] Preparation:
[0061] ① Treatment of raw and auxiliary materials: Dissolve sodium hydroxide and meglumine in 20 g of purified water, add 50 g of ethanol, and then add the prescribed amount of topicastat, and stir until completely dissolved; pass the rest of the auxiliary materials through a standard sieve of 60 mesh or above for later use.
[0062] ② Weigh the diluent, binder and 70g sodium starch glycolate according to the prescription and mix evenly, add the above liquid to make a soft material
[0063] ③ Wet granulation: take the above-mentioned soft material and pass it through a 2...
Embodiment 3
[0069] Prescription (specification: 60mg):
[0070] Topicastat 60.0 g
[0071] Microcrystalline Cellulose 120.0 g
[0072] Mannitol 120.0 g
[0073] Crospovidone 120.0 g
[0074] Sodium hydroxide 6.0 g
[0075] Meglumine 20.0 g
[0076] Hypromellose 6.0 g
[0077] Magnesium stearate 4.0 g
[0078] Micronized silica gel 6.0 g
[0079] A total of 1000 pieces were made
[0080] Preparation:
[0081] ① Treatment of raw and auxiliary materials: Dissolve sodium hydroxide and meglumine in 30g of purified water, add 80g of ethanol, and then add the prescribed amount of topicastat, and stir until completely dissolved; pass the rest of the auxiliary materials through a standard sieve of 60 mesh or above for later use.
[0082] ② Weigh the diluent, adhesive and 100g crospovidone according to the prescription and mix evenly, add the above liquid to make a soft material
[0083] ③ Wet granulation: take the above-mentioned soft material and pass it through a 24-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com