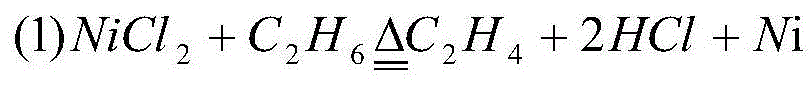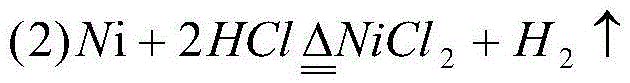Method for continuously preparing ethylene by using ethane
A technology for producing ethane and ethylene, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of difficult handling, low added value of hydrochloric acid, increased cost, etc., and achieve single-pass conversion rate and selectivity improvement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Continuous production of ethylene from ethane:
[0032] (1) Mix 1.0mol ethane with 1.0mol NiCl 2 reaction, the reaction temperature is controlled at 550°C, and the reaction time is 500s, that is, C 2 h 4 Mixed gas with HCl, solid NiCl 2 is reduced to Ni;
[0033] (2) Use the NiCl from the subsequent cycle 2 The C obtained by the solution absorption step (1) reaction 2 h 4HCl in mixed gas with HCl to a hydrochloric acid concentration of 25wt%, NiCl in solution 2 Crystallized out, filtered to get NiCl 2 Solid and hydrochloric acid solution; NiCl obtained by filtration 2 The temperature generated by the subsequent step (1) is at a temperature of about 500° C. 2 h 4 Dry with HCl mixed gas to get 1.0mol NiCl 2 You can return to step (1) for recycling;
[0034] (3) See Table 1 for the main components of the gas phase after removing HCl, and after further rectification and purification, 0.89mol of ethylene with a purity of 99.9% was obtained;
[0035] (4) The hydr...
Embodiment 2
[0040] Continuous production of ethylene from ethane:
[0041] (1) Mix 1.0mol ethane with 1.5mol NiCl 2 reaction, the reaction temperature is controlled at 650°C, and the reaction time is 180s, that is, C 2 h 4 Mixed gas with HCl, solid NiCl 2 is reduced to Ni;
[0042] (2) Use the NiCl from the subsequent cycle 2 The C obtained by the solution absorption step (1) reaction 2 h 4 HCl in mixed gas with HCl to saturation, NiCl in solution 2 Crystallized out, filtered to get NiCl 2 Solid and saturated hydrochloric acid solution; NiCl obtained by filtration 2 The temperature generated by the subsequent step (1) is at a temperature of about 650° C. 2 h 4 Dry with HCl mixed gas to get 1.5mol NiCl 2 You can return to step (1) for recycling;
[0043] (3) See Table 2 for the main components of the gas phase after removing HCl. After further rectification and purification, 0.91 mol of ethylene with a purity of 99.2% was obtained.
[0044] (3) react the saturated hydrochloric...
Embodiment 3
[0049] Continuous production of ethylene from ethane:
[0050] (1) Mix 1.0mol ethane with 2.0mol NiCl 2 reaction, the reaction temperature is controlled at 750°C, and the reaction time is 10s, that is, C 2 h 4 Mixed gas with HCl, solid NiCl 2 is reduced to Ni;
[0051] (2) Use the NiCl from the subsequent cycle 2 The C obtained by the solution absorption step (1) reaction 2 h 4 HCl in mixed gas with HCl to saturation, NiCl in solution 2 Crystallized out, filtered to get NiCl 2 Solid and saturated hydrochloric acid solution; NiCl obtained by filtration 2 The temperature generated by the subsequent step (1) is at a temperature of about 750° C. 2 h 4 Dry with HCl mixed gas to get 2.0mol NiCl 2 You can return to step (1) for recycling;
[0052] (3) The main components of the gas phase product after removing HCl are shown in Table 3. After further rectification and purification, 0.94 mol of ethylene with a purity of 98.8% was obtained.
[0053] (4) react the saturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com