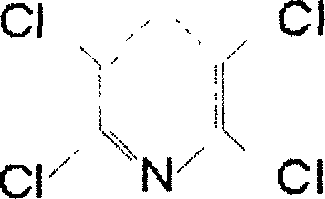
Process for preparing symmetric tetrachloro pyridine
A technology of tetrachloropyridine and chloropyridine is applied in the preparation field of symmetrical tetrachloropyridine, can solve the problems of low yield of symmetrical tetrachloropyridine, high reaction temperature and high production cost, achieves large industrial practical value and simple process , The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 325.6g of 2,6-dichloropyridine, 90.8g of 2-chloropyridine, chlorine Fe 20g, heat until the material melts, turn on the stirrer, continue heating to 170°C, start to feed chlorine gas, keep the temperature and pressure in the primary reactor at about 200°C and 0.04Mpa respectively, and the secondary reactor And the temperature and pressure in the third-stage reactor are about 200°C and about 0.075Mpa, and the appropriate material reflux velocity is controlled, and at the same time, the tail gas in the third-stage reactor is controlled to be free of unreacted chlorine. When the content of symmetrical tetrachloropyridine in the material in the primary reactor reaches 40%, keep other reaction conditions unchanged, but control the temperature and pressure in the primary reactor at about 215°C and 0.06Mpa respectively; And when the content of symmetrical tetrachloropyridine in the material in the primary reactor reaches 80%, it is necessary to control the temperature and p...
Embodiment 2
[0025] The equipment and feeding method are the same as in Example 1, but the initial feeding amount in the reactor is changed to 2,6-dichloropyridine 444g, ferric chloride 20g, and the operation mode is the same as in Example 1. When the primary reactor reaches the end of the reaction , to obtain 670 g of the reaction mixture material, which contains 97.01% of the chlorinated product. GC analysis showed that it contained 97.24% of symmetrical tetrachloropyridine, 0.89% of 2,3,6-trichloropyridine, 1.78% of pentachloropyridine, and 0.09% of other chloropyridine. The conversion rate of symmetrical tetrachloropyridine is 97.14%.
Embodiment 3
[0027]The equipment and feeding method are the same as in Example 1, but the initial feeding amount in the reactor is changed to 485.2 g of polychloropyridine mixture (containing 3-chloropyridine 7.06%, 2-chloropyridine 4.68%, 3,5-dichloropyridine 6.10%, 2,6-dichloropyridine 67.11%, 2,3,5-trichloropyridine 9.39%, 2,3,6-trichloropyridine 5.66%), iron trichloride 22g, the operation method is the same as in Example 1, when a When the first-stage reactor reaches the end of the reaction, 738.8 g of the reaction mixture material can be obtained, which contains 97.02% of the chlorinated product. GC analysis showed that it contained 96.92% of symmetrical tetrachloropyridine, 0.94% of 2,3,6-trichloropyridine, 2.07% of pentachloropyridine, and 0.07% of other chloropyridine. The conversion rate of symmetrical tetrachloropyridine is 97.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com