Synthesis gas preparing low carbon mixed alcohol catalyst and method of preparing the same
A technology of low-carbon mixed alcohols and catalysts, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problem of low selectivity, achieve high selectivity, high activity, and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
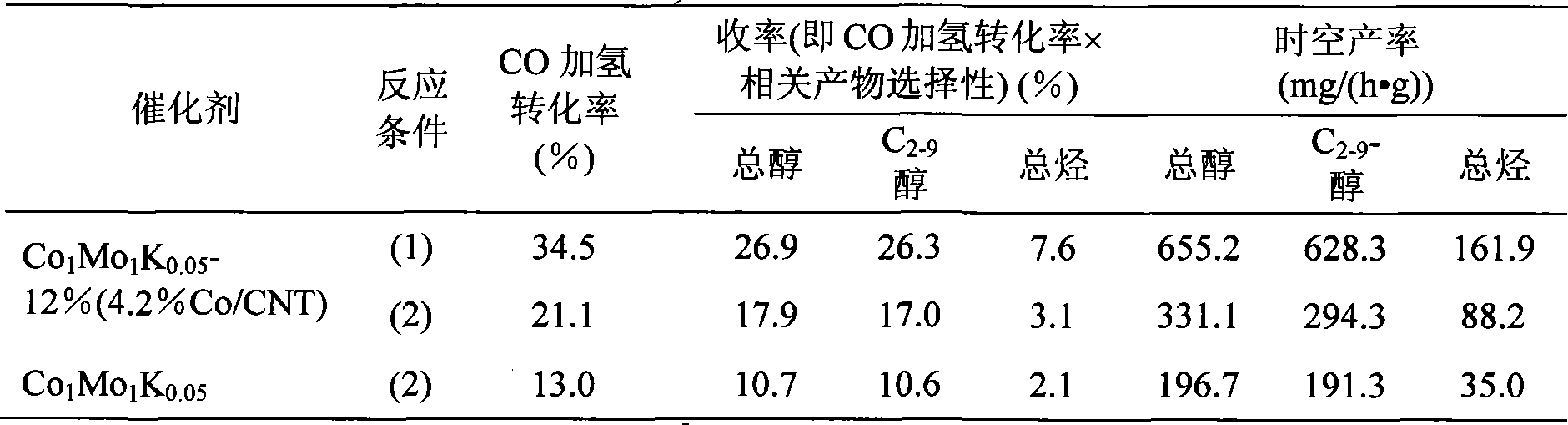
[0021] Weigh 0.662g of cobalt acetate (CoAc 2 4H 2 O, the purity is AR grade) into a 100mL glass cup filled with 50mL ethylene glycol (purity AR grade), stirred until the cobalt acetate is completely dissolved, added 2.106g of purified CNT, and after ultrasonic treatment for 30min, it was Place in a microwave oven (2450MHz, 720W); microwave for 2min, take out the suspension, stir for 10s, reset it into the microwave oven and heat at the same power for 1min, repeat the above stirring / microwave heating operation once, and the precipitate after cooling is suction filtered and removed. After washing with ion water and drying at 110°C, metal Co-modified CNTs were obtained, and the stoichiometric formula was determined to be 4.2% Co / CNT through chemical analysis.
[0022] At 80°C, will contain 7.514gCo(NO 3 ) 2 ·6H 2 O and 4.558g (NH 4 ) 6 Mo 7 o 24 4H 2 The aqueous solution of O (purity is all AR grade) is constant velocity, and dropwise added to the glass container pre-in...
Embodiment 2
[0029] At a temperature of 80°C, it will contain 7.514gCo(NO 3 ) 2 ·6H 2 O and 4.558g (NH 4 ) 6 Mo 7 o 24 4H 2 The aqueous solution of O (purity is AR grade) is constant velocity, and dropwise added to the glass container pre-installed with 0.550g of pure CNT not modified by Co for co-precipitation reaction, vigorously stirred and adjusted to keep the pH value of the solution equal to 5 After 4 hours, stop heating and let it cool down naturally. After 3 hours, stop stirring and let it stand overnight. The precipitate is filtered by suction, washed with deionized water, washed until the filtrate is neutral, and dried at 110°C for 5 hours. Pure N 2 Roast at 500°C for 6 hours in the atmosphere, and then use the isovolumic impregnation method to contain 0.0892gK 2 CO 3 The aqueous solution impregnated and loaded on the calcined precipitate, dried at 110°C for 3h, pure N at 400°C 2 Roasting in the atmosphere for 4h, the stoichiometric formula is Co 1 Mo 1 K 0.05 - Catal...
Embodiment 3
[0032] At a temperature of 80°C, it will contain 7.514gCo(NO 3 ) 2 ·6H 2 O and 4.558g (NH 4 ) 6 Mo 7 o 24 4H 2 The aqueous solution of O (the purity of which is all in AR grade) is constant velocity, and is added dropwise into a glass container pre-installed with 0.400g CNT for co-precipitation reaction. Stir vigorously and adjust and keep the pH value of the solution equal to 5. After 4h, stop heating and let the Cool down naturally, stop stirring after 3 hours and let it stand overnight. The precipitate is suction filtered, washed with deionized water, and washed until the filtrate is neutral, dried at 110°C for 5 hours, pure N 2 Roast at 500°C for 6 hours in the atmosphere, and then use the isovolumic impregnation method to contain 0.0892gK 2 CO 3 The aqueous solution impregnated and loaded on the calcined precipitate, dried at 110°C for 3h, pure N at 400°C 2 Roasting in the atmosphere for 4h, the stoichiometric formula is Co 1 Mo 1 K 0.05 - Catalyst (oxidation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com