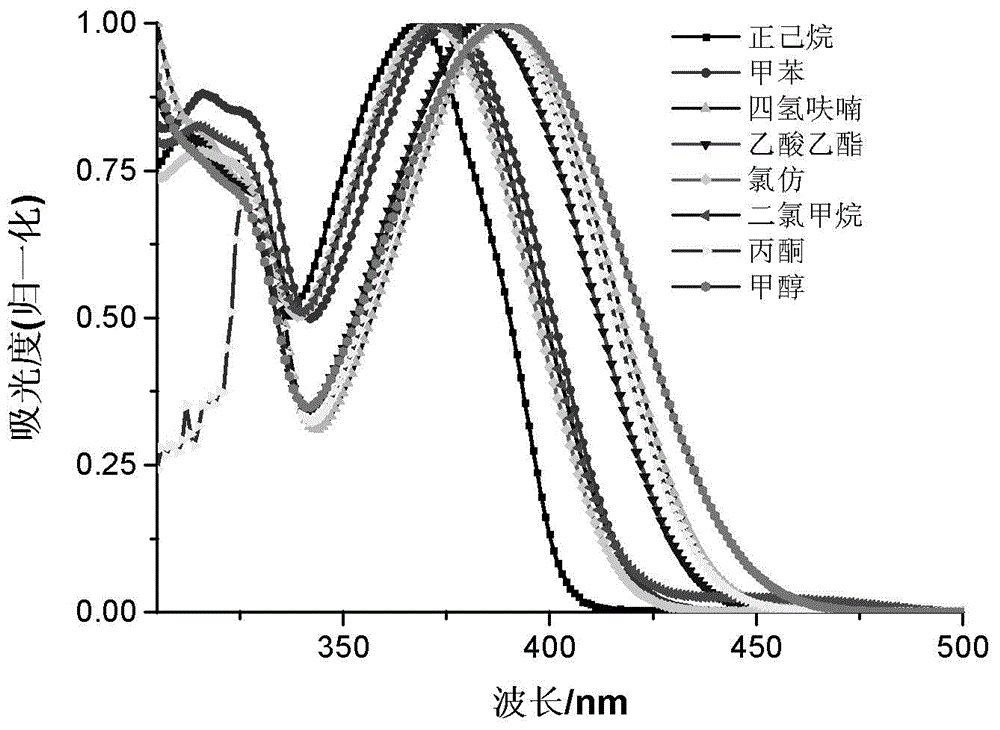
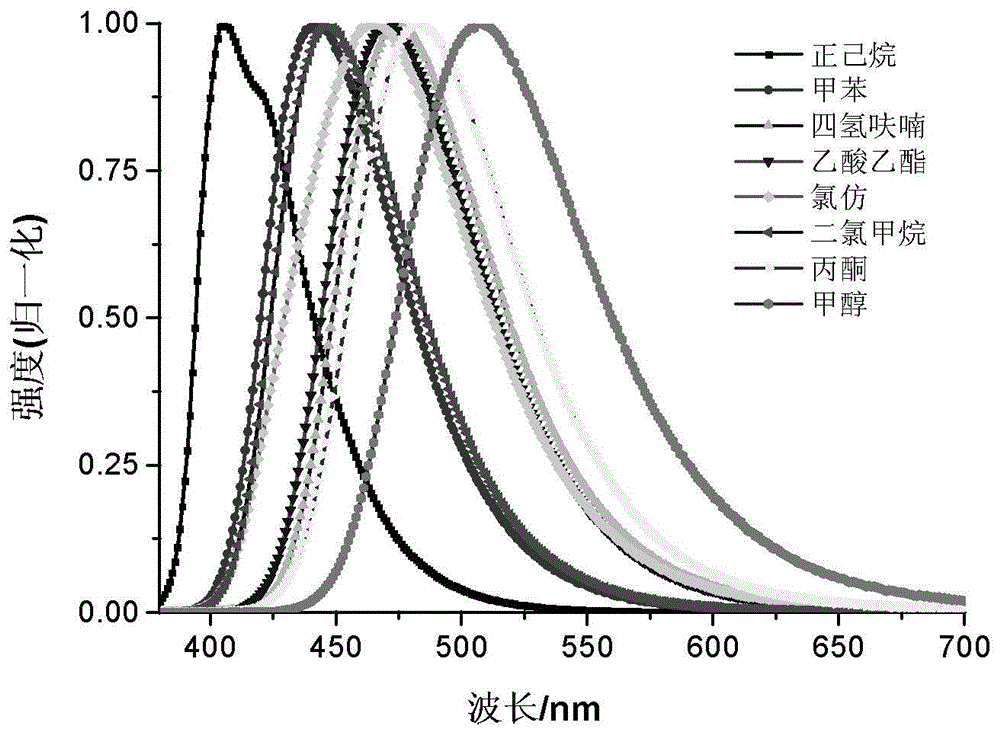
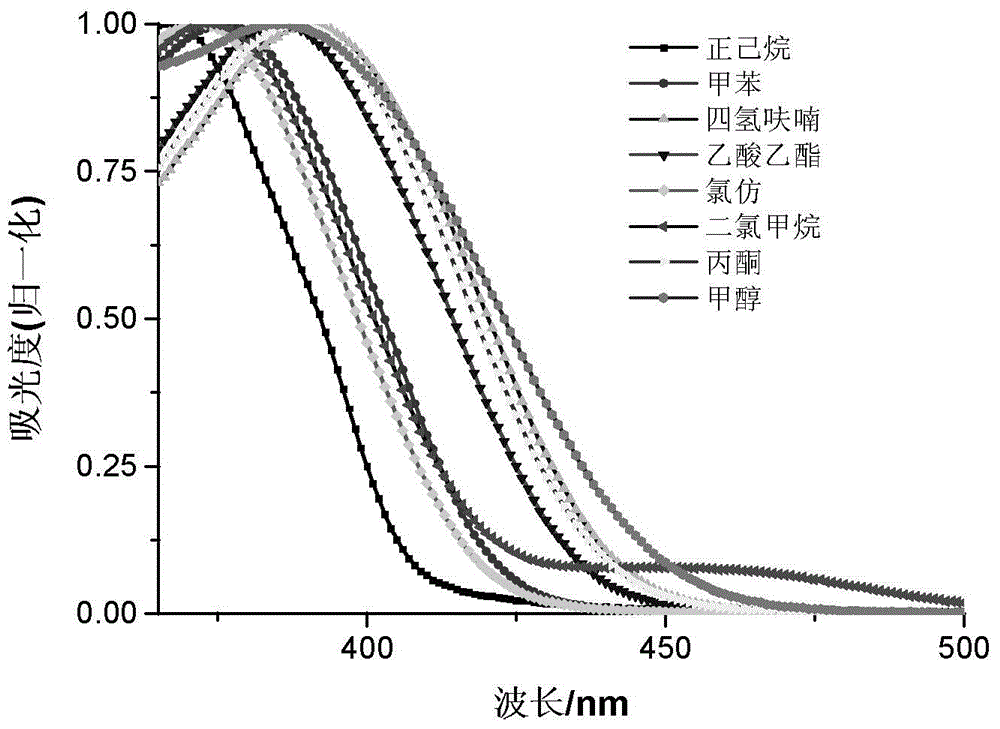
Novel quinoline dye capable of being used as Golgi apparatus organelle probe
A phenyl and naphthyl technology, applied in the field of novel quinoline dyes, can solve the problems of inconvenience, numerous probe synthesis steps, etc., and achieve the effects of low cost, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of two quinoline dyes in chloroform:
[0045] Synthetic steps of Quinoline1:
[0046]
[0047] 1.00 g (4.63 mmol) of 4,4,4-trifluoro-1-phenyl-1,3-butanedione was dissolved in 50 ml of chloroform, and 0.50 g (4.63 mmol) of m-phenylenediamine was added thereto. The reaction system was immersed in an oil bath under N 2 Under protection, stir at reflux at 80°C for about 10 hours. Subsequently, the organic solvent was removed by rotary evaporation under reduced pressure, and the obtained crude product was recrystallized in chloroform, washed with n-hexane, and dried in vacuo to obtain Quinoline1 (1.17 g, 88%) as light grass green needle-like crystals. Structural characterization of Quinoline1: 1 H NMR (300MHz, CDCl 3 )δ8.14(m,2H,4-2'-ArH),7.93(m,1H,5-H),7.89(s,1H,3-H),7.52(m,3H,4-3', 4',5'-ArH),7.39(d,J=2.2Hz,1H,8-H),7.09(dd,J=9.0,2.4Hz,1H,6-H),4.22(broad s,7- NH 2 ,2H).MS(HRMS):m / z calcdforC 16 h 12 f 3 N 2 + 289.09526, found 289.09399.
[0048] Th...
Embodiment 2
[0055] Carry out Combs quinoline synthesis method in water:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com