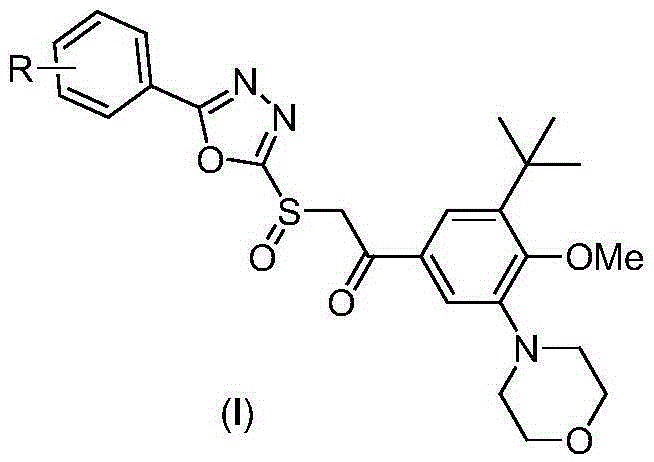
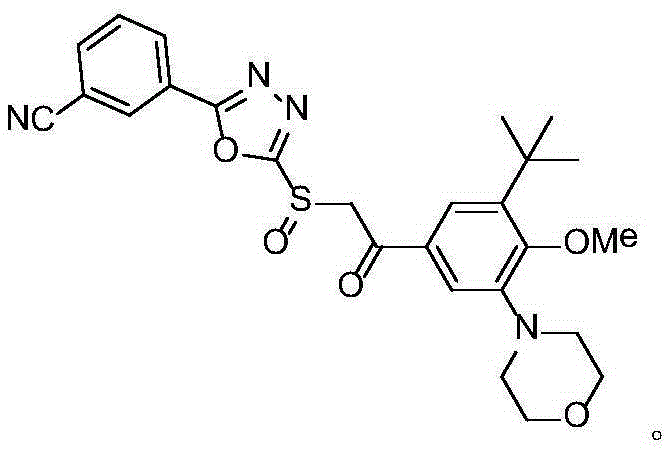
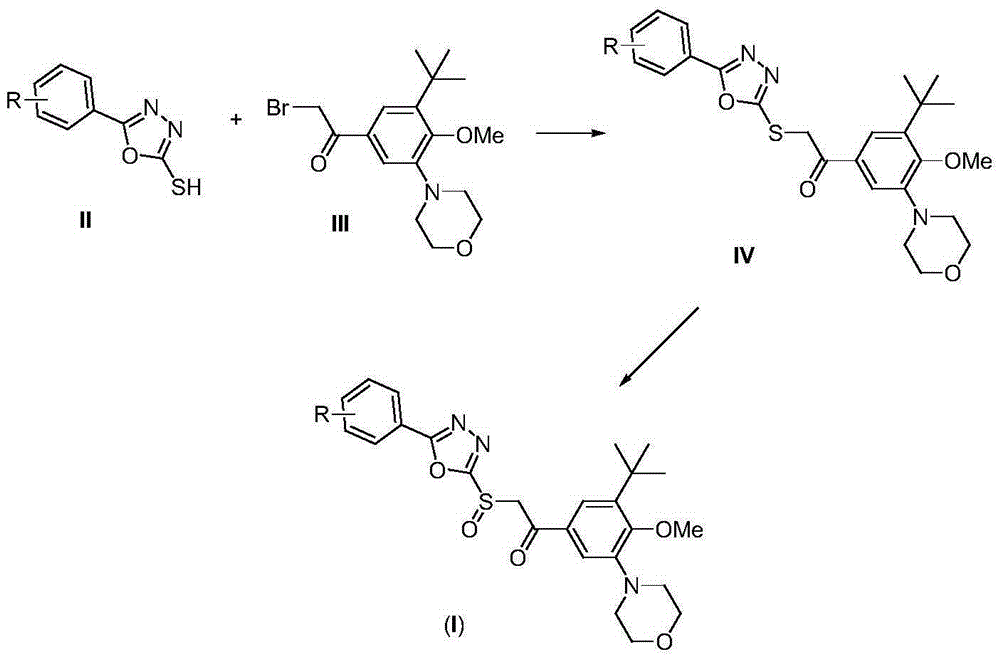
A class of oxadiazole sulfoxide compounds containing cyanobenzene, its preparation method and use
A compound and drug technology, applied in the field of drugs related to thrombosis, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] A. Synthesis of IV-1
[0026] 2.03 g (10 mmol) of compound II-1, 3.70 g (10 mmol) of compound III and 4.15 g (30 mmol) of solid potassium carbonate were stirred overnight in 20 mL of ethanol. The reaction mixture was poured into 200 mL of ice water, stirred, adjusted to pH=4 with concentrated hydrochloric acid, extracted with 50 mL×3 dichloromethane, combined organic phases, washed with brine, dried over anhydrous sodium sulfate, and evaporated the solvent on a rotary evaporator. The obtained residue was purified by column chromatography to obtain pure product IV-1, white solid, MS, m / z=515 ([M+Na] + ).
[0027] B. Synthesis of I-1
[0028] 2.46g (5mmol) of compound IV-1 was dissolved in 25mL of dichloromethane, stirred at -10°C, and 3.13g (20mmol) of m-chloroperoxybenzoic acid (mCPBA) was slowly added. After the reaction mixture was stirred at this temperature for 1 hour, stirring was continued overnight at room temperature. The reaction mixture was pou...
Embodiment 2-3
[0030] According to the method of Example 1, the following compounds with general formula I were synthesized.
[0031]
[0032] Among them, Example 4 is a comparative compound (still a brand new structure discovered by the applicant), to fully illustrate the pharmacological effect.
Embodiment 5
[0033] Example 5 In vitro platelet aggregation inhibition test
[0034] Pharmacological tests of substances were performed in TRAP (thrombin receptor activating peptide)-induced platelet aggregation in 96-well plates. Add 3.13% sodium citrate solution to the syringe in advance, then draw 20mL of blood from healthy volunteers, centrifuge at 1500g for 20 minutes, separate the platelet-rich plasma (PRP) and wash it with 1μL PGE1 solution (500μg / mL ethanol solution) / mLPRP for treatment. After incubation at room temperature for 5 minutes, they were centrifuged at 1200 g for 20 minutes to remove leukocytes. Transfer the leukocyte-free PRP to 15 mL PP tubes in batches at 5 mL / portion, and centrifuge at 3600 g to pellet the platelets. Then, decant the upper layer of plasma, resuspend the platelet pellet from 5 mL of PRP in 1 mL of Tyrode (120 mM NaCl, 2.6 mM KCl, 12 mM NaHCO3, 0.39 mM NaH2PO4, 10 mM HEPES, 0.35% BSA, 5.5 mM glucose, pH=7.4) and adjust to Platelet count of 3×105 / μL....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com