Preparation method of 2,5-furandicarboxylate
A technology of furandicarboxylic acid and furandiformaldehyde, applied in the direction of organic chemistry, etc., can solve the problems of equipment corrosion, low unit production capacity, low raw material concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This embodiment prepares FDCA according to the following steps:
[0027] Add 0.5mmol (62mg) of 2,5-DFF to 0.5mL of 70% tert-butanol peroxy aqueous solution, stir and react at 110°C for 6h, then cool naturally to room temperature to obtain the reaction product, and centrifuge the reaction product The precipitate was separated, washed with 5 mL of water and dried at 105°C to obtain 70 mg of white solid, which was the target product 2,5-furandicarboxylic acid. The molar yield of the product was 90% after testing.
Embodiment 2
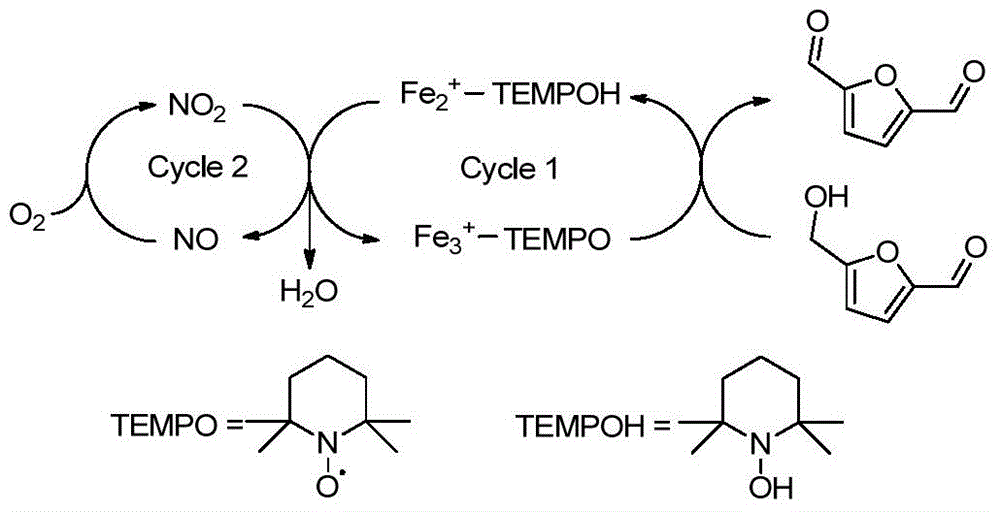
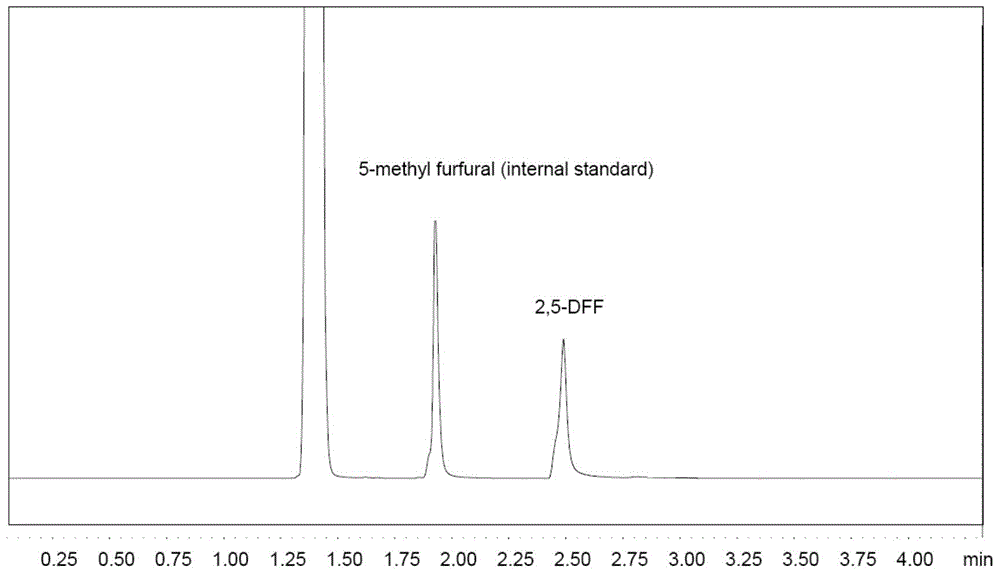
[0029] a. 5-HMF (63mg, 0.5mmol) was added to 2mL of dichloroethane, and then TEMPO (7.8mg, 0.025mmol, 5mol%), Fe(NO 3 ) 3 9H 2 O (10.1mg, 0.025mmol, 5mol%) and NaCl (1.5mg, 0.025mol, 5mol%) were stirred and reacted at room temperature in air for 4h to obtain a reaction mixture containing 2,5-furandicarbaldehyde; GC test results such as figure 1 shown, from figure 1 It can be seen that the product 2,5-furandicarbaldehyde (internal standard: 5-methylfurfural) was successfully obtained in this step.
[0030] b. Add 0.5 mL of 70% tert-butanol peroxy aqueous solution to the reaction mixture, stir and react at 110°C for 6 hours, then cool naturally to room temperature to obtain the reaction product, centrifuge the reaction product to obtain a precipitate, add 5 mL The precipitate was washed with water and dried at 105° C. to obtain 62 mg of white solid, which is the target product FDCA. The molar yield of the product was 80% after testing.
Embodiment 3
[0032] This embodiment prepares FDCA according to the following steps:
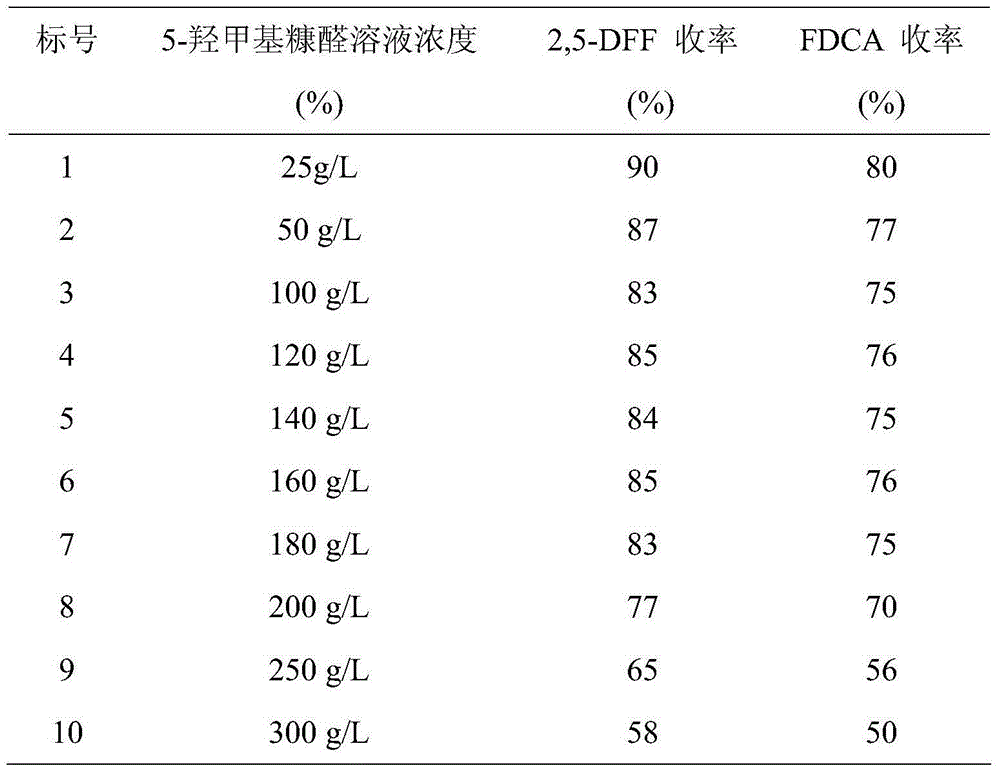
[0033] a. Add 5-HMF into 2mL of dichloroethane, and the prepared mass concentrations are 25g / L, 50g / L, 100g / L, 120g / L, 140g / L, 160g / L, 180g / L, 200g / L The 5-hydroxymethyl furfural solution of L, 250g / L, 300g / L, then add catalyzer tetramethyl piperidine nitroxide (the amount of its substance is 5% of 5-HMF), catalyst activator Fe(NO 3 ) 3 9H 2 O (the amount of its substance is 5% of 5-HMF) and additive NaCl (the amount of its substance is 5% of 5-HMF), stirred and reacted at room temperature in air for 4h to obtain a reaction mixture containing 2,5-DFF solution; the reaction mixture was tested, and the yield of 2,5-DFF was shown in Table 1.
[0034] b. Add 0.5 mL of 70% tert-butanol peroxy aqueous solution to the reaction mixture, stir and react at 110°C for 6 hours, then cool naturally to room temperature to obtain the reaction product, centrifuge the reaction product to obtain a precipitate, add 5 mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com