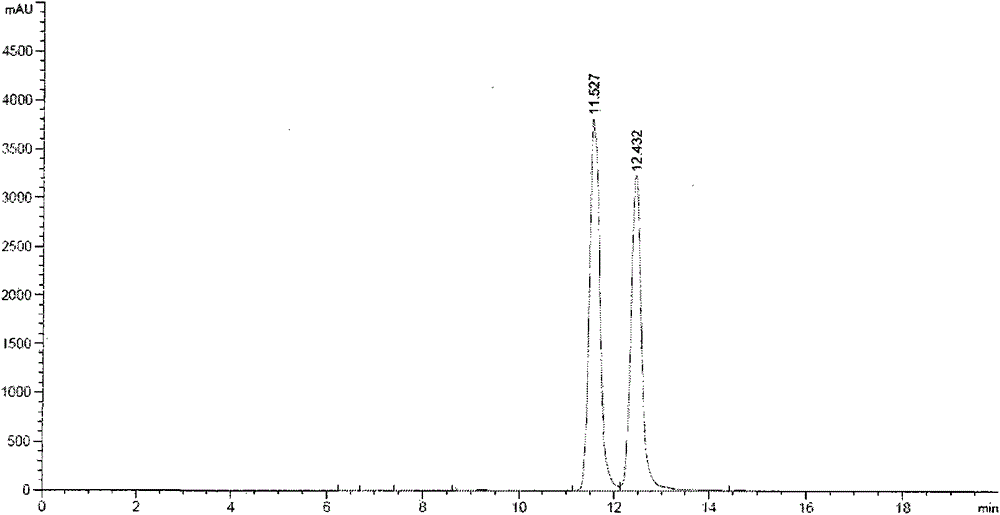
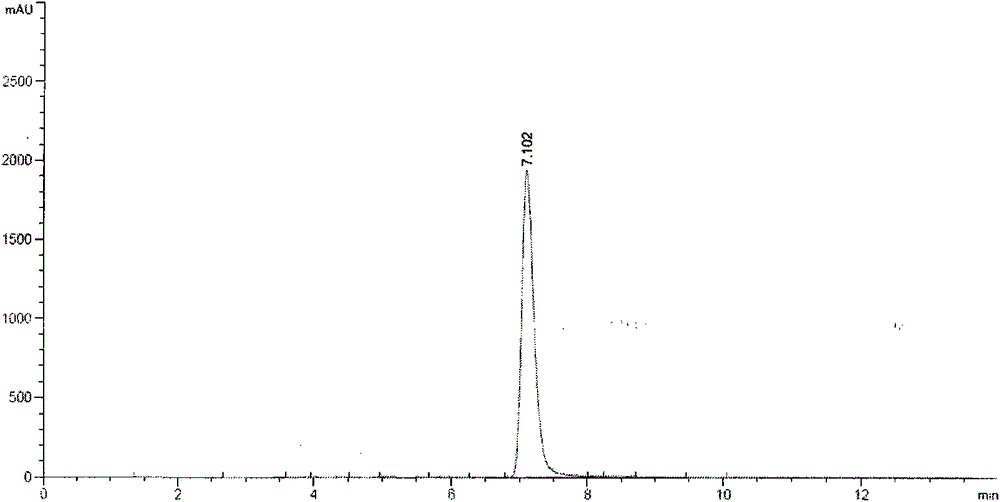
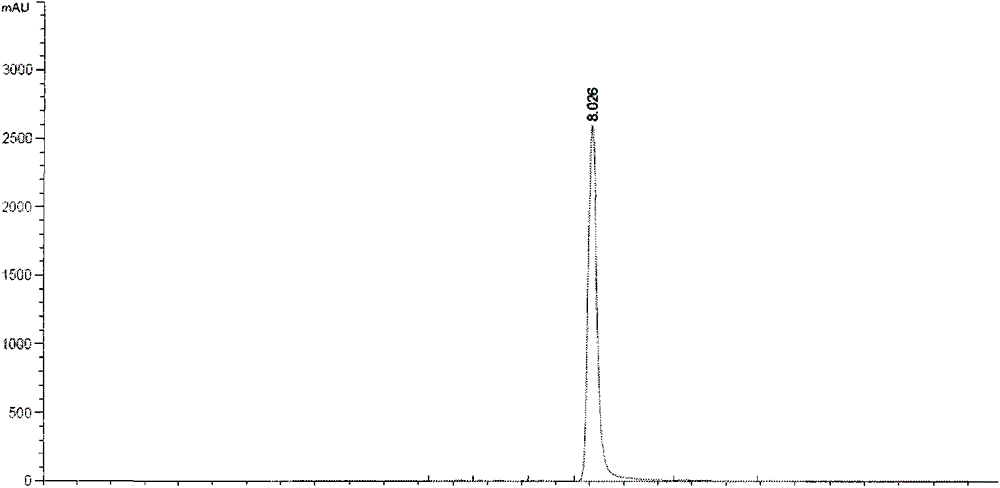
Method for simultaneous preparation of rhamnocitrin and rhamnazin chemical reference substances
A technology of rhamnetin and rhamnetin, which is applied in the direction of organic chemistry, etc., can solve the problems of no rhamnetin and rhamnetin reference substance supply, and no rhamnetin and rhamnetin chemical reference substances, etc. The effect of low separation cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Petroleum ether degreasing: crush 10 kg of blue skyflower into a coarse powder, heat and reflux 7 times with 6 times the amount of petroleum ether at 60-90° C., 1 hour each time. Filter, and evaporate the residue of the blue skyflower to dryness with petroleum ether.
[0027] (2) Ethyl acetate extraction: Add 5 times the amount of ethyl acetate to the degreased N. cerevisiae residue and heat to reflux for 6 times, 1 hour each time. The ethyl acetate extracts were combined, the solvent was recovered, and dried under vacuum at 60°C for 24 hours to obtain 96.1 g of extract.
[0028] (3) Silica gel column chromatographic separation: the dried ethyl acetate part extract was separated by silica gel column chromatography. Take 60g of the ethyl acetate extract and dissolve it in 2 times of absolute ethanol, mix it with 100-200 mesh column chromatography silica gel at a ratio of 1:2, and dry it in vacuum at a constant temperature of 50°C for 12 hours. Take 900g of fresh sil...
Embodiment 2
[0031] (1) Petroleum ether degreasing: crush 10 kg of blue skyflower into a coarse powder, heat and reflux 6 times with 8 times the amount of petroleum ether at 60-90° C., 1 hour each time. Filter, and evaporate the residue of the blue skyflower to dryness with petroleum ether.
[0032] (2) Ethyl acetate extraction: Add 6 times the amount of ethyl acetate to the degreased N. cerevisiae residue and heat to reflux for 6 times, 1 hour each time. The ethyl acetate extracts were combined, the solvent was recovered, and dried under vacuum at 60°C for 24 hours to obtain 100 g of extract.
[0033] (3) Silica gel column chromatographic separation: the dried ethyl acetate part extract was separated by silica gel column chromatography. Take 60g of the ethyl acetate extract and dissolve it in 2 times of absolute ethanol, mix it with 200-300 mesh column chromatography silica gel at a ratio of 1:2, and dry it in vacuum at a constant temperature of 50°C for 12 hours. Take 900g of fresh sili...
Embodiment 3
[0036] (1) Petroleum ether degreasing: crush 10 kg of blue skyflower into a coarse powder, heat and reflux 8 times with 6 times the amount of petroleum ether at 60-90° C., 1 hour each time. Filtration, and evaporate the residue of the blue skyflower to dry petroleum ether.
[0037] (2) Ethyl acetate extraction: Add 7 times the amount of ethyl acetate to the degreased N. radix residue and heat to reflux for 6 times, 1 hour each time. The ethyl acetate extracts were combined, the solvent was recovered, and dried under vacuum at 60°C for 24 hours to obtain 98.1 g of extract.
[0038] (3) Chromatographic separation of polyamide: the dried ethyl acetate extract was separated by polyamide column chromatography. Take 60g of the ethyl acetate extract and dissolve it in 2 times absolute ethanol, mix it with 100-200 mesh polyamide at a ratio of 1:1.5, and dry it in vacuum at a constant temperature of 50°C for 12 hours. Take 1500g of pretreated polyamide and pack it into a column , fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com