Aquilaria sinensis leaf extract as well as preparation method and application thereof
The technology of the leaves and extracts of A. chinensis, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, can solve the problems of toxic and side effects of the body, and achieve simple preparation methods, excellent anti-inflammatory activity, and good anti-inflammation Analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
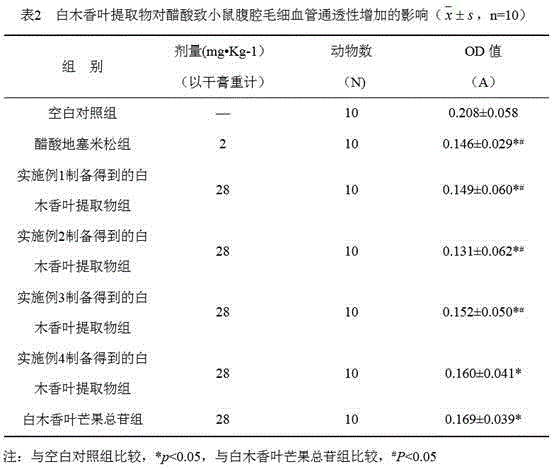
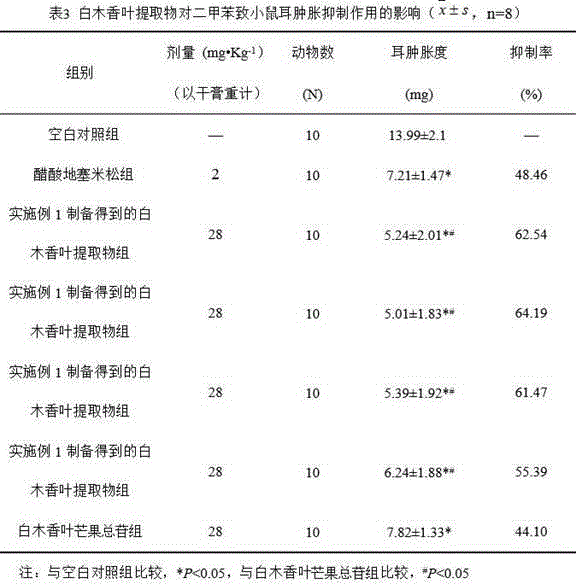
[0025] Weigh 1 kg of dry Akiras sinensis leaf powder and place them in a round-bottomed flask, add 10 times the amount of 70% ethanol, reflux 3 times, 1 hour each time, filter, combine the filtrate, and concentrate the filtrate until it has no alcohol smell; pass through AB- 8 macroporous resin columns, control the flow rate to 2BV / h, first use 5 times the column volume (5BV) of water to elute the macroporous resin to remove impurities; then use 3 times the column volume (60% ethanol) to elute the macroporous resin, collect Concentrate and dry the part eluted with ethanol to obtain the extract of Akiras sinensis leaves.
[0026] After testing, the extract contains 6% of 2-O-a-rhamnoside-4,6,4'.-trihydroxybenzophenone, 5% of 3-C-glucoside-2,4,6, 4'.-tetrahydroxybenzophenone, 40% mangiferin, 6% 3.5-C-glucoside-2,4,6,4'.-tetrahydroxybenzophenone, 14% 5,4 -Dimethoxy-7-hydroxyflavone, 13% acetin-7,4'-dimethyl ether.
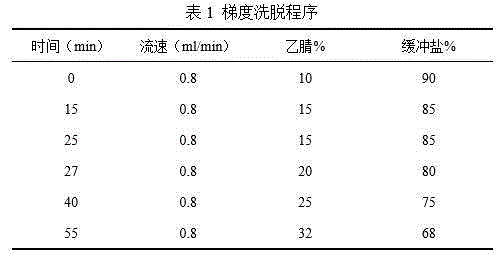
[0027] The detection method of above-mentioned each compositio...
Embodiment 2
[0031] Weigh 1 kg of dry Akiras sinensis leaf powder and place them in a round-bottomed flask, add 10 times the amount of 70% ethanol, reflux 3 times, 1 hour each time, filter, combine the filtrate, and concentrate the filtrate until it has no alcohol smell; pass through AB- 8 macroporous resin columns, control the flow rate to 2BV / h, first use 5 times the column volume (5BV) of water to elute the macroporous resin to remove impurities; then use 3 times the column volume of 50% ethanol to elute the macroporous resin, collect Concentrate and dry the part eluted with ethanol to obtain the extract of Akiras sinensis leaves.
[0032] After testing (the detection method is the same as in Example 1), the extract contains 8% of 2-O-a-rhamnoside-4,6,4'.-trihydroxybenzophenone, 10% of 3-C-glucose Glycoside-2,4,6,4'.-tetrahydroxybenzophenone, 45% mangiferin, 8% 3.5-C-glucoside-2,4,6,4'.-tetrahydroxybenzophenone Ketone, 12% 5,4-dimethoxy-7-hydroxyflavone, 10% apigenin-7,4'-dimethyl ethe...
Embodiment 3
[0034] Weigh 1 kg of dry Akiras sinensis leaf powder and place them in a round-bottomed flask, add 10 times the amount of 70% ethanol, reflux 3 times, 1 hour each time, filter, combine the filtrate, and concentrate the filtrate until it has no alcohol smell; pass through AB- 8 macroporous resin columns, control the flow rate to 2BV / h, first use 5 times the column volume (5BV) of water to elute the macroporous resin to remove impurities; then use 3 times the column volume (40% ethanol) to elute the macroporous resin, collect Concentrate and dry the part eluted with ethanol to obtain the extract of Akiras sinensis leaves.
[0035] After testing (the detection method is the same as in Example 1), the extract contains 10% of 2-O-a-rhamnoside-4,6,4'.-trihydroxybenzophenone, 12% of 3-C-glucose Glycoside-2,4,6,4'.-tetrahydroxybenzophenone, 52% mangiferin, 9% 3.5-C-glucoside-2,4,6,4'.-tetrahydroxybenzophenone Ketones, 4% 5,4-dimethoxy-7-hydroxyflavone, 6% apigenin-7,4'-dimethyl ether...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com