Synthesis method of cilnidipine
A technology of cilnidipine and a synthesis method is applied in the field of calcium antagonist cilnidipine synthesis and calcium antagonist preparation, and can solve the problems of low yield and the like, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
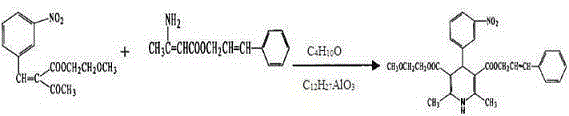
[0015] The preparation method of the catalyst aluminum isobutoxide in the present invention is: in a 250ml there-necked bottle equipped with a reflux condenser and a thermometer, add excess isobutanol, an appropriate amount of aluminum particles, and a small amount of initiator, and reflux at a certain temperature until the aluminum The pellets were completely dissolved, and the excess isobutanol was removed under reduced pressure.
[0016] The cinnamyl β-aminocrotonate in the present invention is prepared by the preparation method described in the patent 200810038932.X, and then recrystallized.
Embodiment 1
[0018] Place 100 g of commercially available 2-(3-nitrobenzylidene) 2-methoxyethyl acetoacetate and 82 g of cinnamyl β-aminocrotonate in a reaction kettle, and add 250 mL of isobutanol solvent and Aluminum isobutoxide catalyst 0.16g, reflux for 3-4h; cool overnight and filter to obtain yellow crystals; wash with ethanol at 5-10°C, and recrystallize with absolute ethanol to obtain 130.4g of light yellow crystals, cilnidipine refined product, yield 75.3%.
Embodiment 2
[0020] Put 100 g of commercially available 2-(3-nitrobenzylidene) 2-methoxyethyl acetoacetate and 82 g of cinnamyl β-aminocrotonate in a reaction kettle, and add 200 mL of isobutanol solvent and Aluminum isobutoxide catalyst 0.33g, reflux for 3-4h; cool overnight and filter to obtain yellow crystals; wash with ethanol at 5-10°C, and recrystallize with absolute ethanol to obtain 132.0g of fine cilnidipine crystals, yield 76.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com