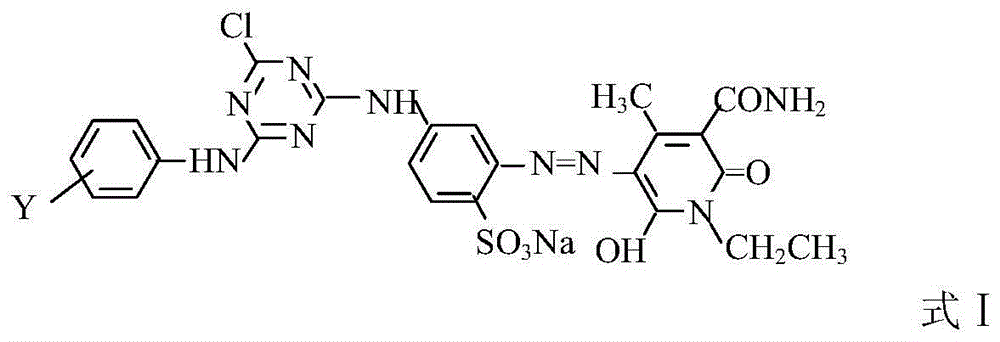
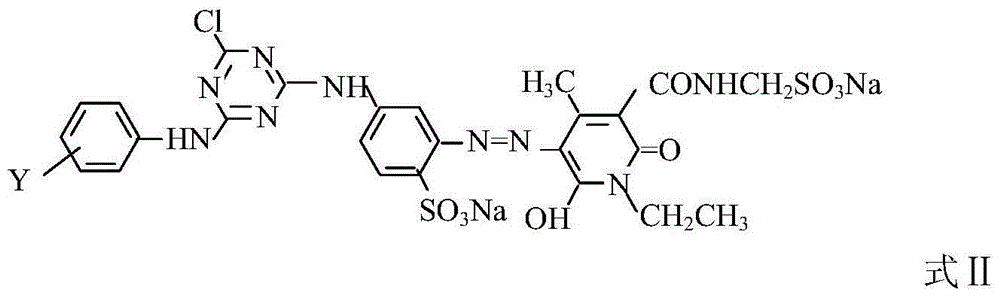
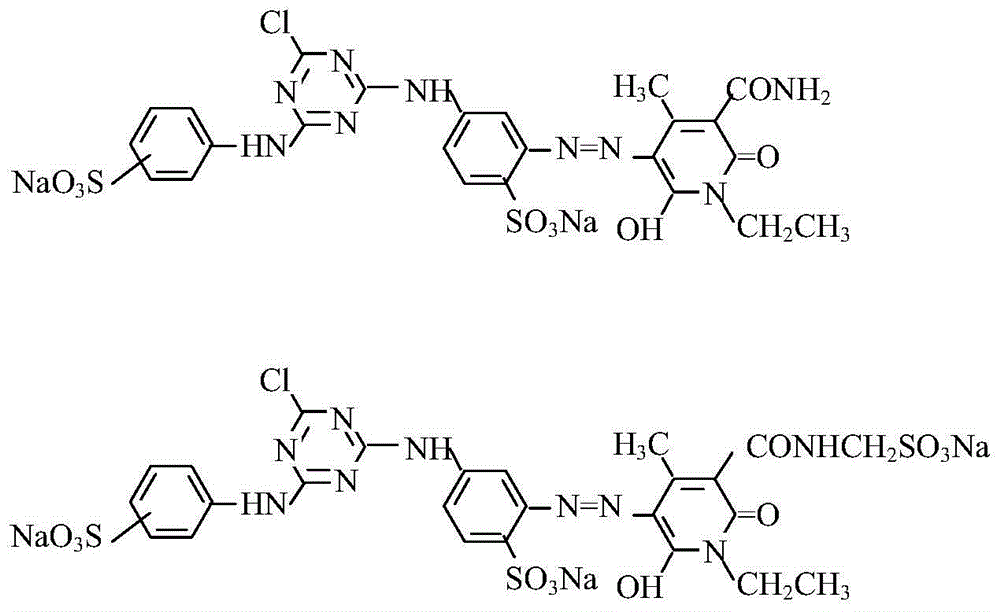
Composite reactive light yellow dye composition
A dye composition, a technology of active bright yellow, applied in the field of dyes, can solve the problems of poor color fixation rate, unsuitable for automatic sizing system, unfavorable to environmental protection, etc., and achieves good printing lifting power, good color and light controllability, and dyeing improvement. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Beat 1.02 moles of cyanuric chloride with ice and water for 1 hour, control the speed for 1 hour, add 1 mole of 2,4-diaminobenzenesulfonic acid solution with a pH value of 6.5 to 7.0 and a concentration of 20%, and control the temperature at 0 to 5 Carry out a condensation reaction at ℃, V=700-750L / 0.1KM, after 2 hours, use 15% soda ash to quickly adjust the pH=7.0-7.5, add 3.5 moles of hydrochloric acid, 1.0 moles of sodium nitrite solution for diazotization, and keep T=0 -10°C, starch KI test paper slightly blue, Congo red blue, after adding, keep the conditions, react for 1 hour, and use sulfamic acid to balance the excess nitrous acid.
[0022] 0.4 moles of N-ethyl-3-carbamoyl-4-methyl-6-hydroxy-2-pyridone and 0.6 moles of N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- 6-Hydroxy-2-pyridone is beaten with water, soda ash to adjust PH=6.0~7.0, after dissolving, add the above-mentioned diazonium salt, soda ash to adjust PH=6.0~7.5, react at 5~10°C for 2~3 hours, measure d...
Embodiment 2
[0024] In Example 1, N-ethyl-3-carbamoyl-4-methyl-6-hydroxyl-2-pyridone and N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- The molar ratio of 6-hydroxy-2-pyridone was changed to 0.5:0.5, the secondary condensate was changed to m-aminobenzenesulfonic acid, and other process conditions remained unchanged to obtain commercial dyes.
Embodiment 3
[0026] In Example 1, N-ethyl-3-carbamoyl-4-methyl-6-hydroxyl-2-pyridone and N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- The molar ratio of 6-hydroxy-2-pyridone was changed to 0.45:0.55, the secondary condensate was changed to m-aminobenzenesulfonic acid, and other process conditions remained unchanged to obtain commercial dyes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| color fastness | aaaaa | aaaaa |
| soaping fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com