Application of chlorogenic acid in preparation of drug for treating choriocarcinoma
A technique for choriocarcinoma and chlorogenic acid, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
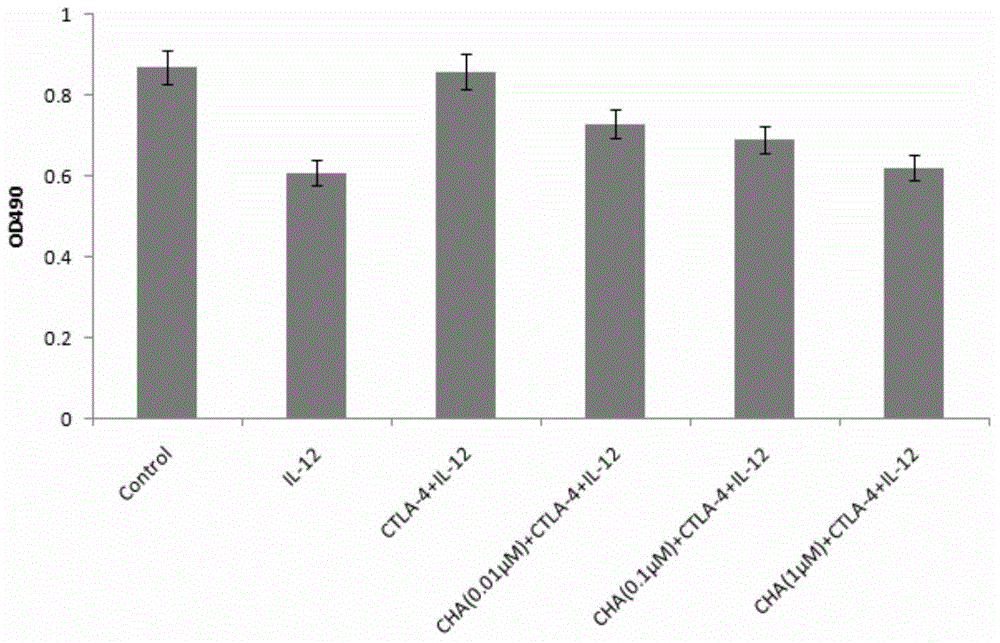
[0029] Example 1 Simulation experiment of anti-tumor effect of chlorogenic acid targeting CTLA-4
[0030] cell culture
[0031] The choriocarcinoma cell line JEG-3 was grown in high-glucose DMEM complete medium containing 10% fetal bovine serum (supplemented with 2mmol / L glutamine, 1mmol / L sodium pyruvate, 100U / ml penicillin and 100U / ml streptomycin In a 37°C, 5% CO2 incubator, the medium was changed every 3 or 3 days. When the cells are about 70% to 80% full, passage with 0.25% trypsin and 0.02% EDTA mixture at a ratio of 1:3.
[0032] MTT colorimetric assay for cell proliferation
[0033] Take the JEG-3 cells in the exponential growth phase according to the initial concentration of 3 × 10 4 / ml inoculated in 96-well plate, and divided into 6 groups: control group, IL-12 group, CTLA-4+IL-12 group, 0.01 μM chlorogenic acid+CTLA-4+IL-12 group, 0.1 μM chlorogenic acid +CTLA-4+IL-12 group and 1μM chlorogenic acid+CTLA-4+IL-12 group at 37°C, 5%CO 2 After culturing in the incu...
Embodiment 2
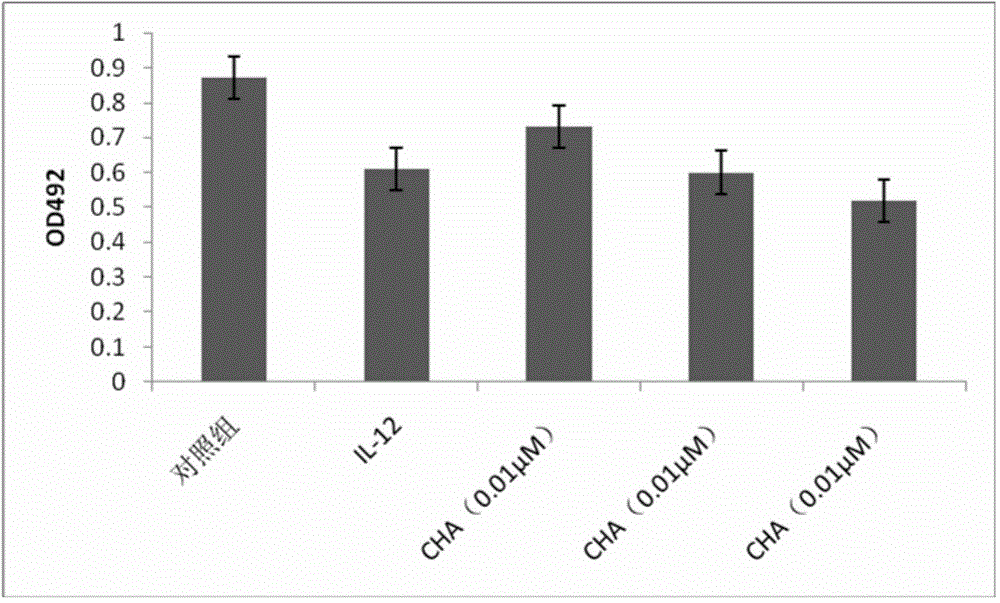
[0040] Effect of Example 2 on the proliferation of JEG-3 choriocarcinoma cells
[0041] cell culture
[0042] The choriocarcinoma cell line JEG-3 was grown in high-glucose DMEM complete medium containing 10% fetal bovine serum (supplemented with 2mmol / L glutamine, 1mmol / L sodium pyruvate, 100U / ml penicillin and 100U / ml streptomycin In a 37°C, 5% CO2 incubator, the medium was changed every 3 or 3 days. When the cells are about 70% to 80% full, passage with 0.25% trypsin and 0.02% EDTA mixture at a ratio of 1:3.
[0043] MTT colorimetric assay for cell proliferation
[0044] Take the JEG-3 cells in the exponential growth phase according to the initial concentration of 3 × 10 4 / ml inoculated in a 96-well plate, and divided into 5 groups: control group, IL-12 group, chlorogenic acid 0.01 μM, 0.1 μM, 1 μM group at 37 ° C, 5% CO 2 After culturing in the incubator for 48 hours, replace it with serum-free medium to continue starvation and synchronization for 24 hours; then add IL-1...
Embodiment 3
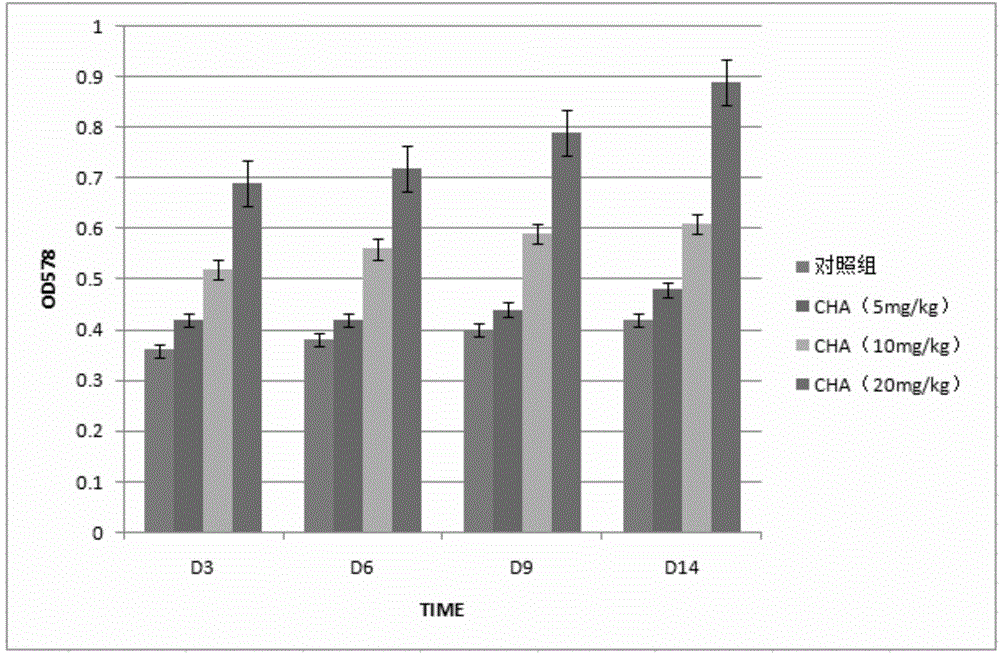
[0051] Example 3 Chlorogenic acid inhibits the activity of CTLA-4
[0052] The inhibitory mechanism of CTLA-4 is realized by mediating the apoptosis of activated T cells. The researchers found that CTLA-4 can mediate Fas-independent T cell apoptosis. After the apoptosis of activated T cells, the number of effector T cells decreases, the secretion of cytokines decreases, and the immune function of the body is naturally suppressed.
[0053] Mouse CTLA-4 model, BALB / c mouse, male, 18-22g. CTLA-430mg / kg dose was injected once, and the establishment of the model was evaluated 14 days later. The next day after the model was successfully established, the animals were randomly divided into groups, weighed, and began to administer drugs. Chlorogenic acid injection (a series of intraperitoneal injections of chlorogenic acid with different concentrations made by dissolving chlorogenic acid in normal saline according to the dosage) The administration volume is 0.2ml per 10g mouse intra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com