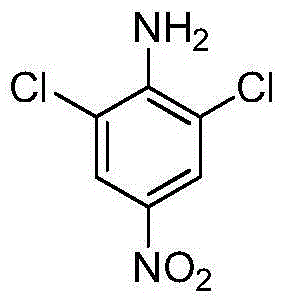
Low-temperature synthesis method of 2,6-dichloro-4-nitroaniline
A technology for nitroaniline and p-nitroaniline, which is applied in the field of low-temperature synthesis of 2,6-dichloro-4-nitroaniline, can solve the problems of reduced concentration of hydrochloric acid solution, generation of waste water, and high production cost, and achieves the cost of raw materials Low energy consumption, less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
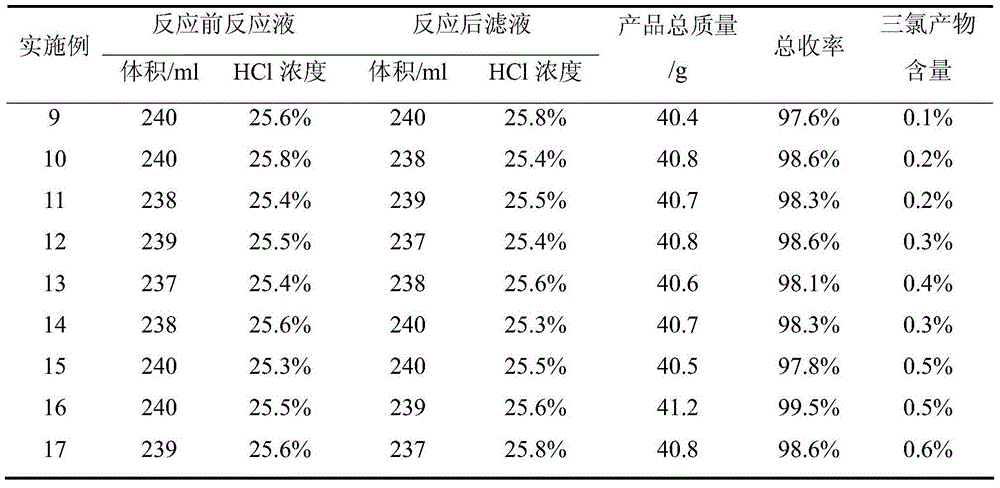
Examples
Embodiment 1
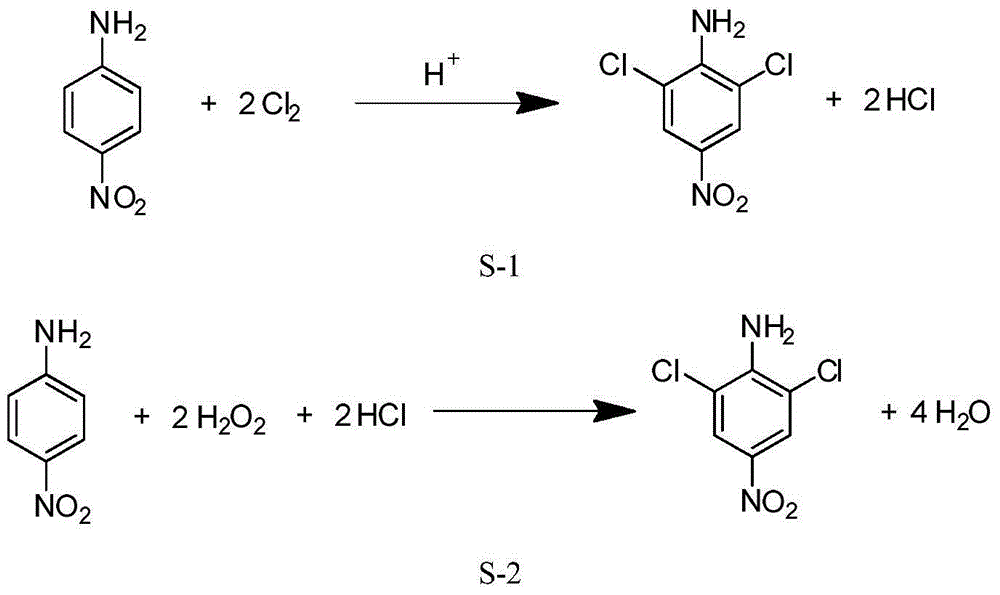
[0033] Embodiment 1, a kind of preparation method of 2,6-dichloro-4-nitroaniline, take p-nitroaniline as raw material, carry out the following steps successively:
[0034] 1), after mixing 140mL of 35wt% concentrated hydrochloric acid (1.6mol) and 100mL water as dilute hydrochloric acid, the concentration of this dilute hydrochloric acid is 25.6wt% (marked value).
[0035] Put 13.8g (0.1mol) of p-nitroaniline (0.1mol) and the above dilute hydrochloric acid into a 500mL three-necked flask, cool to -8°C in a low-temperature tank under mechanical stirring, and control at this temperature, pass in about 0.3mol of chlorine gas, stir, and carry out Chlorination reaction; keep stirring for 2h (at this time, TLC detects that the reaction of raw materials is complete).
[0036] After the reaction, the reaction liquid was left naturally at room temperature, and a pale yellow solid was precipitated, which was filtered to obtain a filter cake of 2,6-dichloro-4-nitroaniline and the filtrat...
Embodiment 2
[0040] Embodiment 2, a kind of preparation method of 2,6-dichloro-4-nitroaniline, take p-nitroaniline as raw material, carry out the following steps successively:
[0041] 1), 110mL of 35% concentrated hydrochloric acid (1.27mol) and 130mL of water are mixed as dilute hydrochloric acid, and the concentration of the dilute hydrochloric acid is 20.8%wt% (marked value).
[0042] Put 13.8g (0.1mol) of p-nitroaniline (0.1mol) and the above-mentioned dilute hydrochloric acid into a 500mL three-necked flask, and under mechanical stirring, cool to -10°C in a low-temperature tank. reaction; insulated and stirred for 1 h (at this time, TLC detected that the reaction of the raw materials was complete).
[0043] After the reaction, the reaction liquid was left naturally at room temperature, and a pale yellow solid was precipitated, which was filtered to obtain a filter cake of 2,6-dichloro-4-nitroaniline and the filtrate (which was the mother liquor of hydrochloric acid). After the filte...
Embodiment 3
[0047] Embodiment 3, a kind of preparation method of 2,6-dichloro-4-nitroaniline, take p-nitroaniline as raw material, carry out the following steps successively:
[0048] 1), after mixing 195mL of 35% concentrated hydrochloric acid (2.2mol) and 45mL of water as dilute hydrochloric acid, the concentration of this dilute hydrochloric acid is 31.2wt% (standard value).
[0049] Put 13.8g (0.1mol) of p-nitroaniline (0.1mol) and the above-mentioned dilute hydrochloric acid into a 500mL three-necked flask, and under mechanical stirring, cool to -6°C in a low-temperature tank. reaction; insulated and stirred for 2h (at this time, TLC detected that the reaction of raw materials was complete).
[0050] After the reaction, the reaction liquid was left naturally at room temperature, and a pale yellow solid was precipitated, which was filtered to obtain a filter cake of 2,6-dichloro-4-nitroaniline and the filtrate (which was the mother liquor of hydrochloric acid). After the filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com