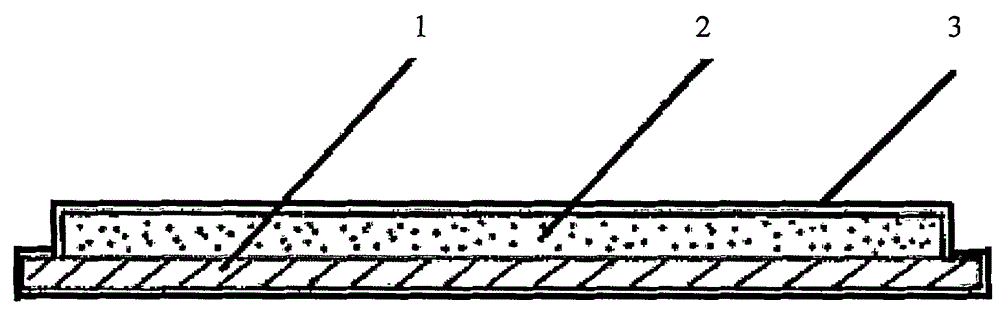
Antiseptic agarose gel plate preparation method
A technology of agarose gel and agarose, which is applied in the field of medicine and health, and can solve the problems of short storage time, trouble, and easy growth of bacteria in agarose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
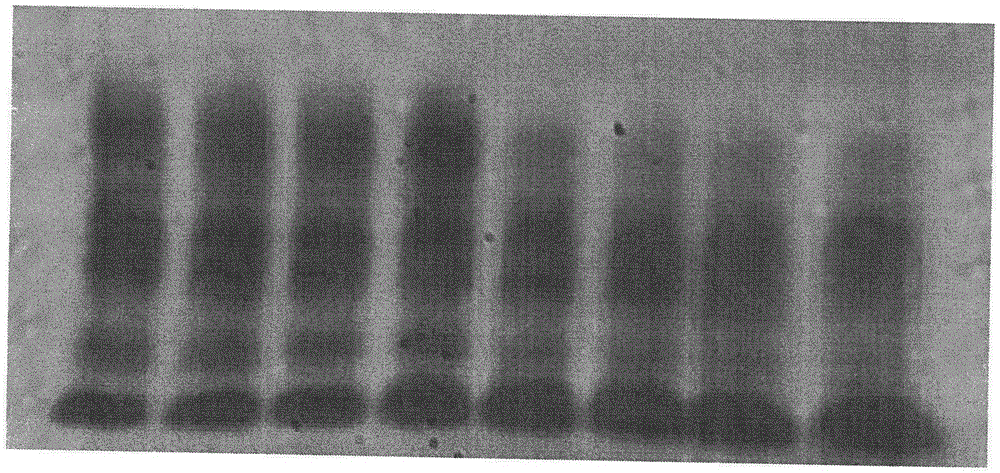
[0058] Example 1 made the same day experiment figure ( image 3 )
[0059] Take a fresh serum sample, use the preservative agarose gel plate made on the same day for protein electrophoresis experiment, and analyze the results:
[0060] swim lane ALB alpha 1 globulin alpha 2 globulin beta globulin gamma globulin 1 64.5 3.6 8.7 12.3 10.8 2 64.8 3.8 9.2 11.7 10.4 3 65.2 4.0 8.7 12.1 10.0 4 65.9 3.9 8.9 11.5 9.8 mean(x) 65.1 3.83 8.9 11.9 10.3 Standard Deviation(s) 0.52 0.15 0.20 0.32 0.38 Coefficient of Variation (CV%) 0.81 3.87 2.31 2.66 3.75
Embodiment 24
[0061] Example 24 ℃ refrigerator preservation 3 months sample experiment figure ( Figure 4 )
[0062] Take a sample and seal the antiseptic agarose gel plate with moisture in the box, moist appearance, sufficient moisture in the agar plate, plump, and no bacterial growth. Take a fresh serum sample, use the sample to do protein electrophoresis experiment, and analyze the results (because there is tailing in lane 4, the result is excluded):
[0063] swim lane ALB alpha 1 globulin alpha 2 globulin beta globulin gamma globulin 1 54.7 4.9 10.2 14.3 16.0 2 54.2 4.3 9.6 14.6 17.3 3 53.4 4.8 10.3 13.2 18.2 5 52.2 4.3 10.2 15.1 18.2 6 54.7 4.0 10.5 12.6 18.2 7 55.6 4.7 9.9 13.6 16.2 mean(x) 54.1 4.5 10.1 13.9 17.4 Standard Deviation(s) 1.19 0.35 0.32 0.93 1.03 Coefficient of Variation (CV%) 2.20 7.83 3.15 6.72 5.94
Embodiment 34
[0064] Example 34 ℃ refrigerator preservation 4 months sample experiment figure ( Figure 5 )
[0065] Take a sample and seal the antiseptic agarose gel plate with moisture in the box, moist appearance, sufficient moisture in the agar plate, plump, and no bacterial growth. Take a fresh serum sample, use the sample to do protein electrophoresis experiment, and analyze the results:
[0066] swim lane ALB alpha 1 globulin alpha 2 globulin beta globulin gamma globulin
[0067] 1 61.2 2.8 7.9 12.4 15.7 2 63.0 2.5 8.1 10.1 16.2 3 60.2 2.8 7.1 13.0 16.8 4 60.7 2.6 7.1 11.7 17.9 5 60.0 2.6 7.2 11.8 18.5 6 62.4 2.8 6.3 11.6 16.9 7 62.1 2.7 7.2 11.4 16.6 8 62.3 3.0 6.5 11.5 16.8 9 61.7 2.7 8.2 11.9 15.6 10 61.0 2.7 8.4 11.1 16.7 mean(x) 16.46 2.72 7.4 11.65 16.77 Standard Deviation(s) 1.00 0.14 0.72 0.76 0.89 Coefficient of Variation (CV%) 1.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com