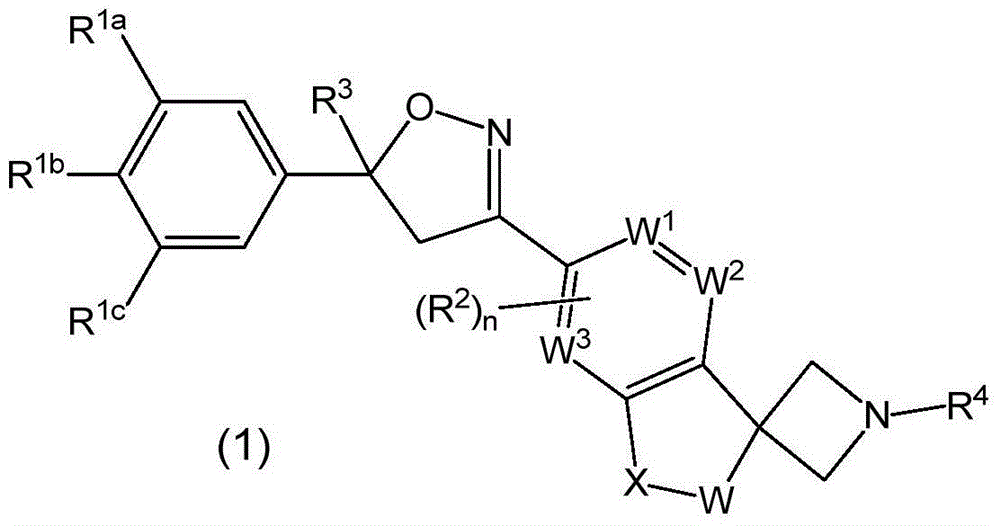
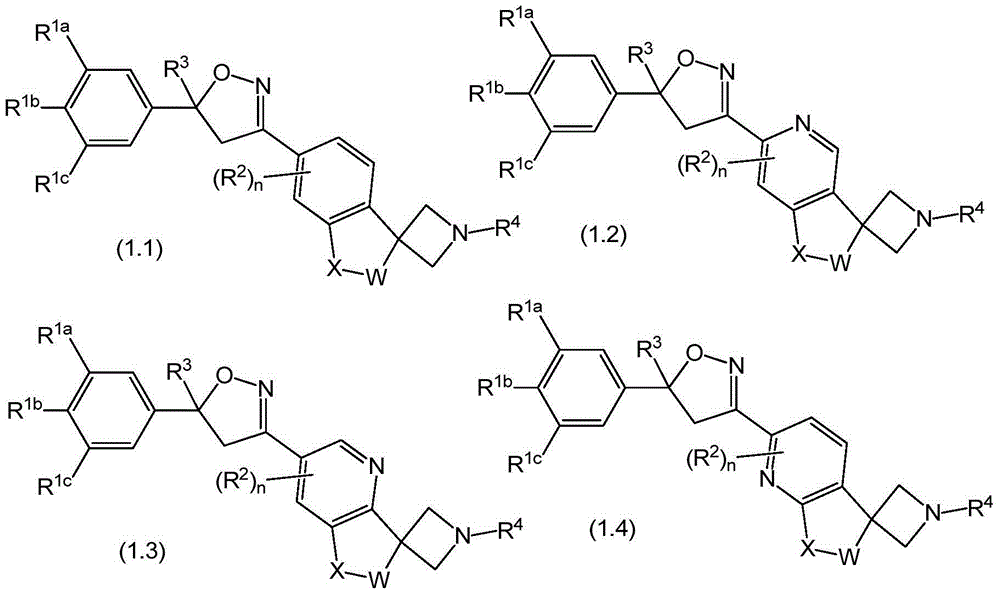
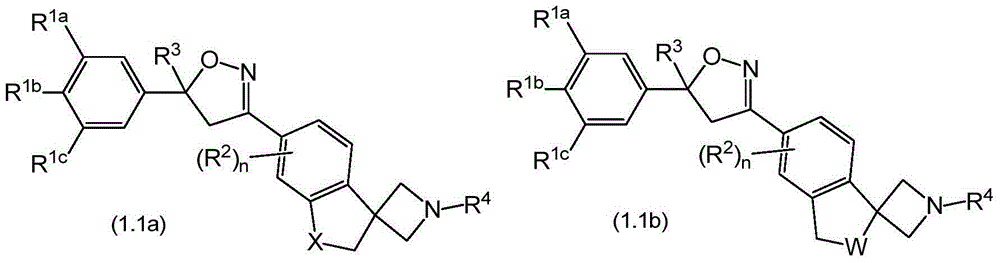
Spirocyclic isoxazolines as antiparasitic agents
A technology of cycloalkyl and stereoisomers, which is applied in the field of manufacturing the spirocyclic isoxazoline derivatives, and can solve problems such as not indicating the range of life cycle stages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0134] In the preparation of compounds of the present invention, protection of remote functional groups of intermediates from undesired reactions can be accomplished with protecting groups. The term "protecting group" or "Pg" refers to a substituent typically used to block or protect a particular functional group while allowing other functional groups on the compound to react. For example, an amine protecting group is an amine-attached substituent that blocks or protects the amine functionality of a compound or intermediate. Suitable amine protecting groups include: 1-tert-butoxycarbonyl (Boc); acyl groups including: formyl, acetyl, chloroacetyl, trichloro-acetyl, o-nitrophenylacetyl, o-nitrophenoxy Acetyl, trifluoroacetyl, acetoacetyl, 4-chlorobutyryl, isobutyryl, o-nitrocinnamoyl, picolinoyl, acyl isothiocyanate, aminocaproyl, benzoyl, etc.; and acyloxy, including: methoxycarbonyl, 9-fluorenyl-methoxycarbonyl, 2,2,2-trifluoroethoxycarbonyl, 2-trimethylsilylethoxycarbonyl, v...
example
[0193] The following examples can be prepared according to the schemes and preparations as presented herein.
[0194] Intermediate 1. tert-butyl 3'-bromo-1H,5'H-spiro[azetidine-3,7'-furo[3,4,b]pyridine]-1-carboxylate
[0195]
[0196] Step 1. 5-Bromo-2-iodo-3-methyl-pyridine
[0197]
[0198] To a stirred solution of 2,5-dibromo-3-methyl-pyridine (20.0 g, 79.7 mmol, 1 eq) in acetonitrile (120 mL) was added acetyl chloride (8.5 mL, 119.5 mmol, 1.5 eq) at room temperature, Then NaI (47.8 gm, 319 mmol, 4 eq) was added. The resulting reaction mixture was refluxed for 16 hours. After complete consumption of starting material, the reaction mixture was quenched with water (200 mL), and extracted with ethyl acetate (3 x 200 mL). The organic layer was washed with aqueous sodium bicarbonate (2 x 300 mL), brine (300 mL), and dried over sodium sulfate. The organic layer was concentrated under reduced pressure to give a brown oil (35.5 g, crude). The crude material was purified ...
example 1
[0210] Example 1. 3'-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl]-1-[(methylsulfonyl Acyl)acetyl]-5'H-spiro[azetidine-3,7'-furo[3,4,b]pyridine]
[0211]
[0212] Step 1. tert-butyl 3'-formyl-1H,5'H-spiro[azetidine-3,7'-furo[3,4,b]pyridine]-1-carboxylate
[0213]
[0214] 3'-Bromo-1H,5'H-spiro[azetidine-3,7'-furo[3,4,b]pyridine]-1-carboxylic acid tert-butyl ester (intermediate To a stirred solution of compound 1, 2g, 5.7mmol, 1eq) in anhydrous THF (30mL) was added n-BuLi (1.0M in hexane, 6.4mL, 7.0mmol, 1.2eq), and at -78°C Stir under nitrogen atmosphere for 10 minutes. After 10 minutes, anhydrous DMF (0.68 mL, 8.79 mmol, 1.5 eq) was added at -78 °C. The resulting reaction mixture was stirred at -20°C under nitrogen atmosphere for 3 hours. After complete consumption of the starting material, the reaction mixture was washed with 10% NH 4 The Cl solution (10 mL) was quenched, diluted with water (50 mL), and extracted with ethyl acetate (2 x 50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com