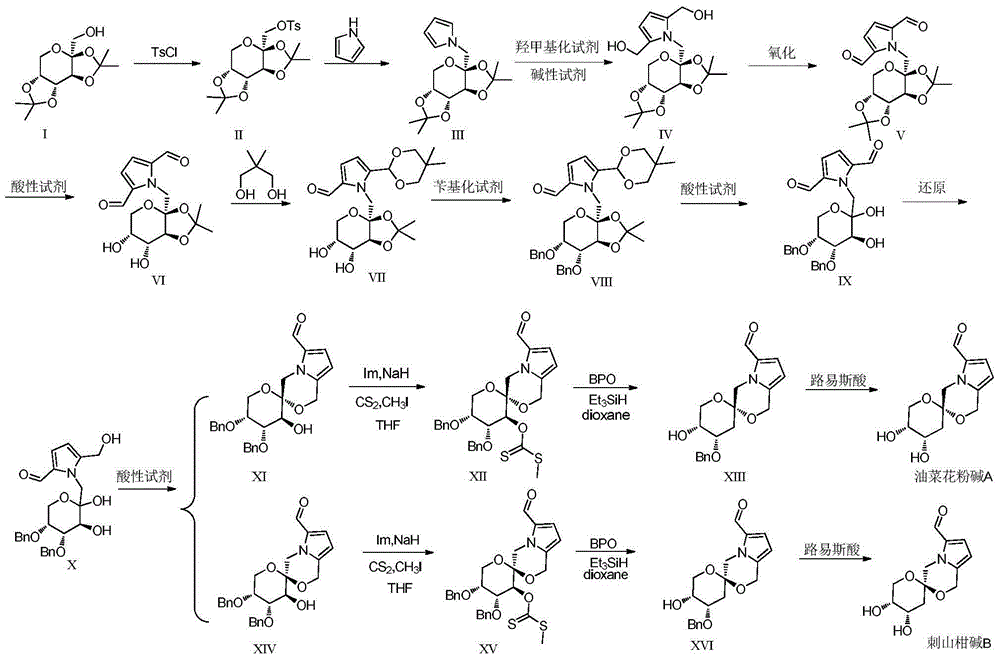
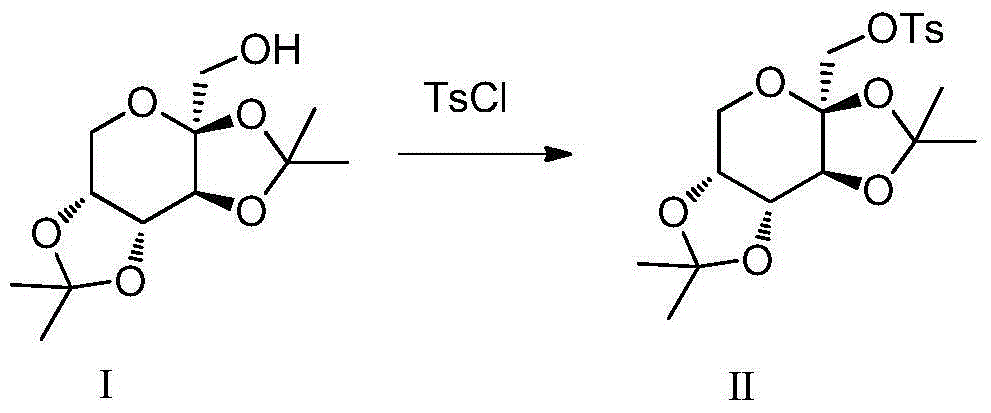
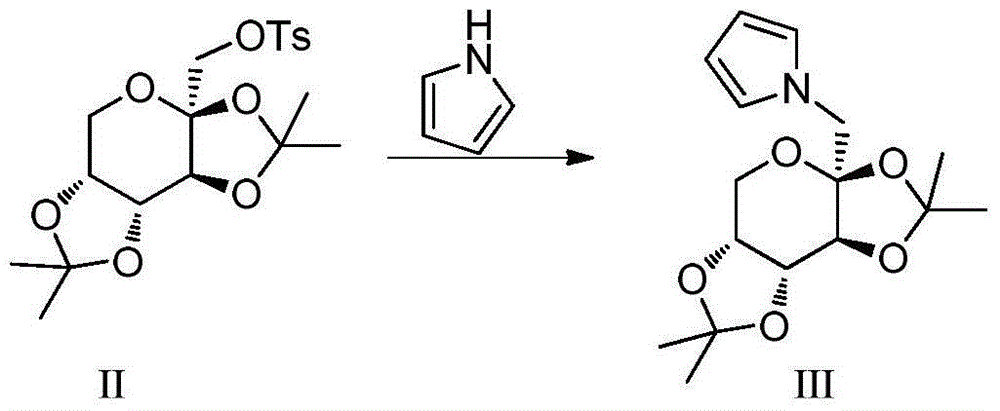
Total synthesis method for rape pollen alkali A and caper alkali B and analogues thereof
A technology of rapeseed pollen and caperine, which is applied to chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high price and large amount of original drug, and achieve low environmental pollution and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The present invention will be described in further detail below in conjunction with the examples, but the embodiments of the present invention are not limited thereto.
[0039] Weigh 15.19g of sodium hydride and put it in a 500mL three-necked flask, dissolve it with 50mL of DMF, measure 10mL of pyrrole, dissolve it with 60mL of DMF, slowly add the DMF solution of pyrrole (about 3 seconds a drop) in the flask under ice bath, after the drop Weigh 30.58g of compound II, dissolve it in 60mL of DMF, slowly add the raw material solution dropwise (about 3 seconds per drop) into a three-necked flask under ice bath, transfer to a 40°C oil bath to react for 1h, raise the temperature of the oil bath to 135°C, React for 4-5 days, TLC (P:E=3:1) detects that the reaction is basically complete, add a small amount of methanol to neutralize NaH until no bubbles are formed, distill off DMF under reduced pressure, extract the reaction solution with ethyl acetate multiple times, and the org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com