Preparation method and application of medicinal aqueous acrylic resin aqueous dispersion and product produced from medicinal aqueous acrylic resin aqueous dispersion
A technology of acrylic resin and dispersion, applied in water dispersion products and its application as polymer pharmaceutical excipients in solid pharmaceutical dosage forms, in the field of preparation of pharmaceutical acrylic resin water dispersion, can solve the problem of high aggregate content, Problems such as poor film formation, adverse effects on acrylic resin polymer structure and performance, etc., to achieve the effect of simple operation process and reduced residual monomer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
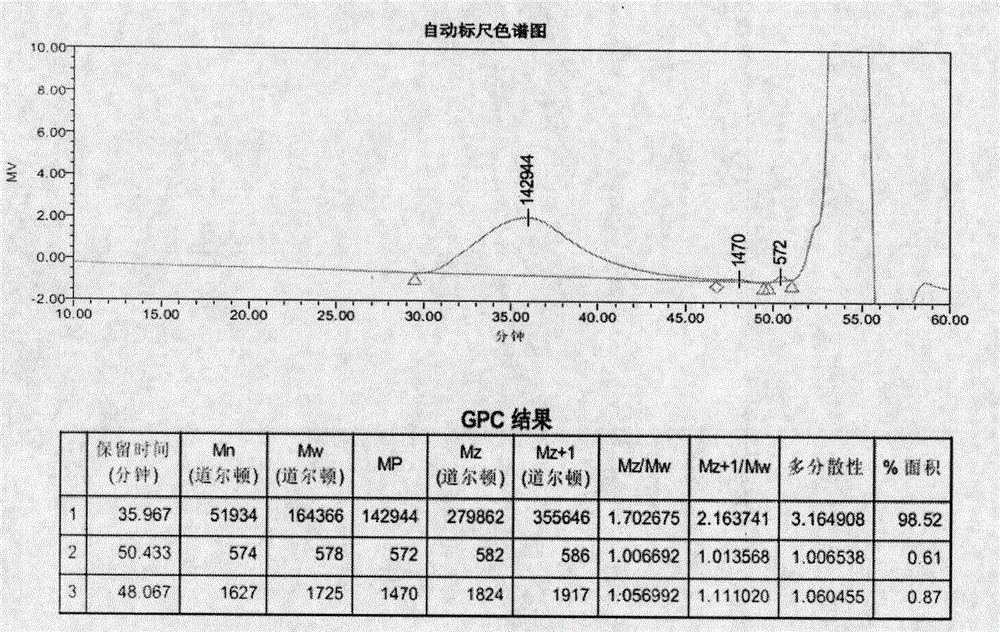
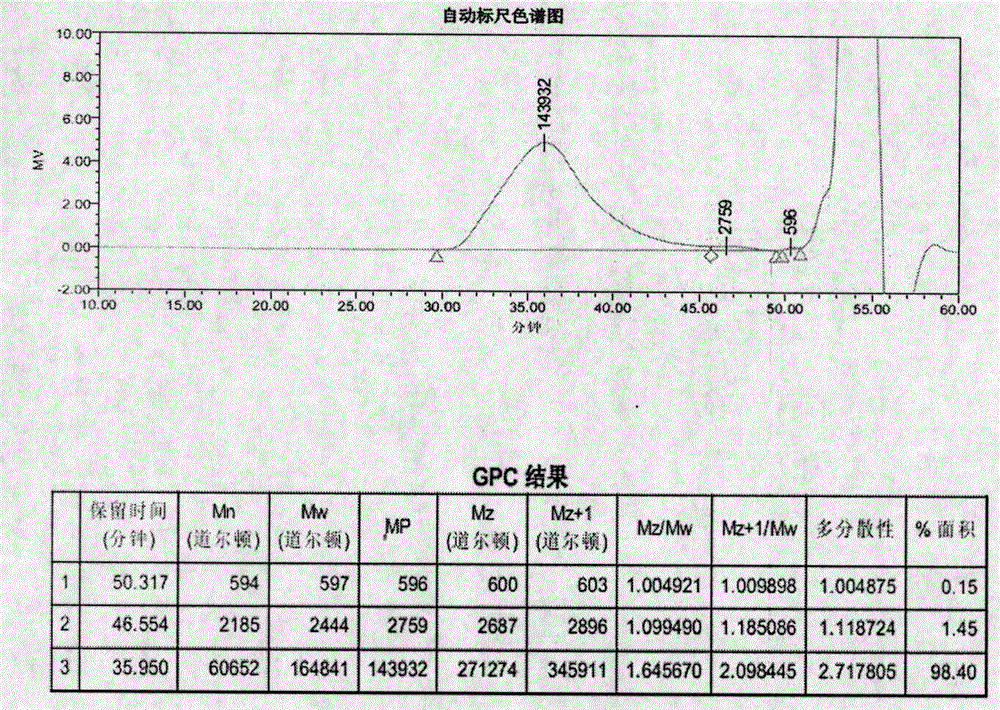
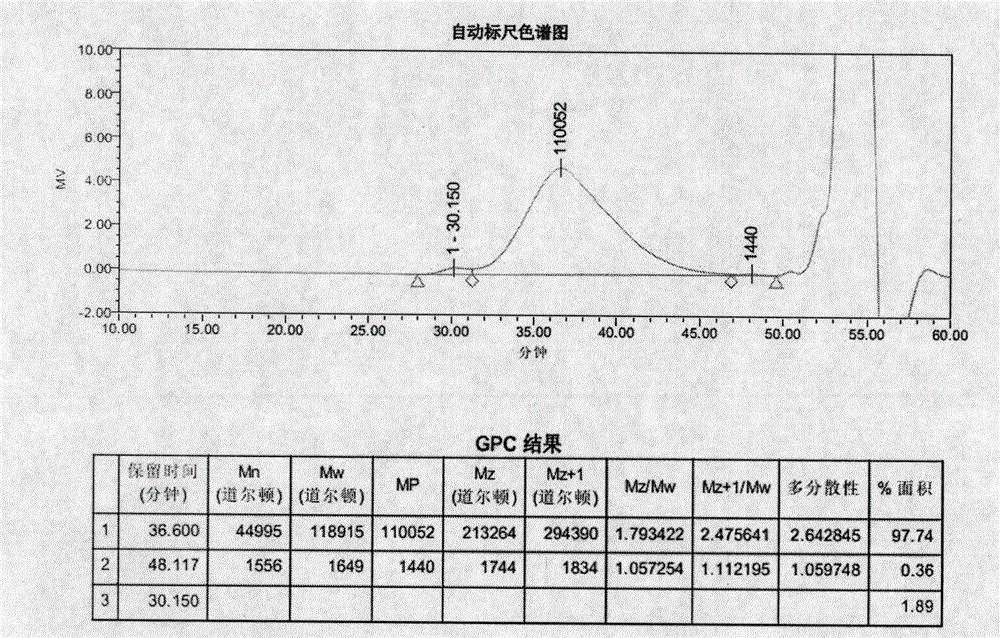
Image
Examples
Embodiment 1
[0107] Example 1 (ME-262)
[0108] Instruments: 100-liter experimental reaction kettle, water bath, paddle stirrer, temperature measuring device and processing control pipeline system for metering and feeding.
[0109] Preparation steps:
[0110] Heating the initial feed liquid to an internal temperature of 75°C, adding 5.6 parts of ammonium persulfate (6%) for 5 minutes, maintaining the temperature at 80°C and metering in 24.4 parts of the feed liquid dropwise, the dripping is completed within 1 hour, and the insulation reaction is 1 Hours, add 2.5 parts of isoascorbic acid (6%) to react for 5 minutes. Cool down to 58-60°C, add 4.6 parts of ferrous sulfate heptahydrate (0.1%) to react for 5 minutes, add 1.7 parts of tert-butyl hydroperoxide (6%) to react for 5 minutes, and drop the remaining dripping feed liquid into the The dripping was finished within 90-120 minutes and the feed liquid I was dripped within 120-150 minutes, and then the reaction was continued for 15 minute...
Embodiment 2
[0128] Example 2 (ME-261)
[0129] Instruments: 1 liter experimental reaction kettle, water bath, paddle stirrer, temperature measuring device and processing control pipeline system for metering and feeding.
[0130] Preparation steps:
[0131] Heat the initial feed solution to an internal temperature of 75°C, add 6.3 parts of ammonium persulfate (6%) and react for 5 minutes, then maintain the temperature at 80°C and add 48.8 parts of the dropwise feed solution dropwise, and finish the drop within 1.5 hours. After 1 hour, add 5.6 parts of isoascorbic acid (3%) and react for 5 minutes. Cool down to 58-60°C, add 4.6 parts of ferrous sulfate heptahydrate (0.1%) to react for 5 minutes, add 1.7 parts of tert-butyl hydroperoxide (6%) to react for 5 minutes, and drop the remaining dripping feed liquid into the The dripping was finished within 90-120 minutes and the feed liquid I was dripped within 120-150 minutes, and then the reaction was continued for 15 minutes. After adding 1....
Embodiment 3
[0149] Example 3 (ME-257)
[0150] Instruments: one 100-liter experimental reaction kettle, water bath, paddle stirrer, temperature measuring device and processing control pipeline system for metering and feeding.
[0151] Preparation steps:
[0152] Heat the initial feed liquid to an internal temperature of 75°C, add 3.3 parts of ammonium persulfate (6%) and react for 5 minutes, then maintain the temperature at 80°C and add 8 parts of the feed liquid dropwise, and finish the dripping within 1 hour. 1 hour, add 2.9 parts of isoascorbic acid (3%) and react for 5 minutes; cool down to 58-60° C., add 4.6 parts of ferrous sulfate heptahydrate (0.15%) and react for 5 minutes, add 5 parts of tert-butyl hydroperoxide ( 3%) after reacting for 5 minutes, drop the remaining dripping feed liquid in 90-120 minutes and feed liquid I in 120-150 minutes, then continue to react for 15 minutes; add 5 parts of tert-butyl After hydrogen peroxide (3%) reacted for 5 minutes, drop feed solution I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com