Preparation method of manganese-base fluorescent powder
A fluorescent powder and manganese-based technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of complicated preparation process and unfavorable display of white light LED, and achieve the effect of low cost and improved display
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of manganese-based fluorescent powder, which is specifically carried out according to the following steps:
[0030] (1) Under normal temperature and pressure conditions, the KMnO 4 , KF is first added to the HF solution, after stirring evenly, add H 2 o 2 Carry out the reaction, and after the reaction is completed, filter with suction, wash with methanol, and dry to obtain the precursor K 2 MnF 5 ·H 2 O; in this step (1), the consumption of each material is KMnO according to molar ratio 4 :KF:H 2 o 2 :HF=1:2:4:200 for configuration;
[0031] (2) The precursor K 2 MnF 5 ·H 2 O, K 2 TiF 6 , KMnO 4, KF is added in the HF solution, stirred evenly and reacted for 40 h, and finally through suction filtration, washed with methanol, and dried; in this step (2), the consumption of each material is K according to the molar ratio 2 MnF 5 ·H 2 O:K 2 TiF 6 : KMnO 4 :KF:HF=1:20:5:5:500 to configure.
[0032] The principle of its preparation re...
Embodiment 2
[0038] A preparation method of manganese-based fluorescent powder, which is specifically carried out according to the following steps:
[0039] (1) Under normal temperature and pressure conditions, the KMnO 4 , KF is first added to the HF solution, after stirring evenly, add H 2 o 2 Carry out the reaction, and after the reaction is completed, filter with suction, wash with ethanol, and dry to obtain the precursor K 2 MnF 5 ·H 2 O; in this step (1), the consumption of each material is KMnO according to molar ratio 4 :KF:H 2 o 2 :HF=1:0.2:2:100 for configuration;
[0040] (2) The precursor K 2 MnF 5 ·H 2 O, K 2 TiF 6 , KMnO 4 , KF is added in the HF solution, stirred evenly and reacted for 10 h, and finally through suction filtration, washed with ethanol, and dried; in this step (2), the consumption of each material is K according to the molar ratio 2 MnF 5 ·H 2 O:K 2 TiF 6 : KMnO 4 :KF:HF=1:0.1:0.5:0.5:100 to configure.
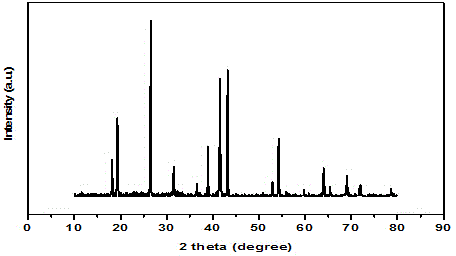
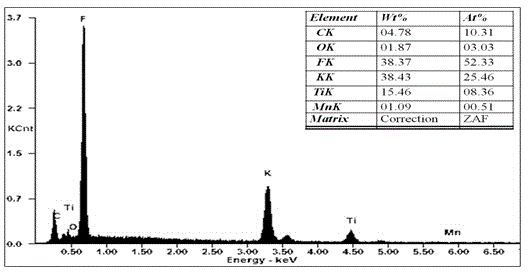
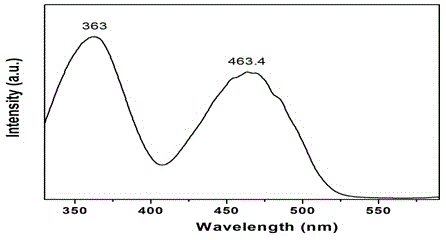
[0041] combined with Figure 5 , 6...
Embodiment
[0043] A preparation method of manganese-based fluorescent powder, which is specifically carried out according to the following steps:
[0044] (1) Under normal temperature and pressure conditions, the KMnO 4 , KF is first added to the HF solution, after stirring evenly, add H 2 o 2 Carry out the reaction, and after the reaction is completed, filter with suction, wash with acetone, and dry to obtain the precursor K 2 MnF 5 ·H 2 O; in this step (1), the consumption of each material is KMnO according to molar ratio 4 :KF:H 2 o 2 :HF=1:1:2:300 for configuration;
[0045] (2) The precursor K 2 MnF 5 ·H 2 O, K 2 TiF 6 , KMnO 4 , KF is added in the HF solution, stirred evenly and reacted for 1 h, and finally through suction filtration, washed with acetone, and dried; in this step (2), the consumption of each material is K according to the molar ratio 2 MnF 5 ·H 2 O:K 2 TiF 6 : KMnO 4 :KF:HF=1:10:4:4:500 to configure.
[0046] combined with Figure 8 , 9 And the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com