Composition and preparation method thereof as well as oral liquid and preparation method of oral liquid
A composition and solution technology, applied in the direction of drug combination, active ingredient of heterocyclic compound, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
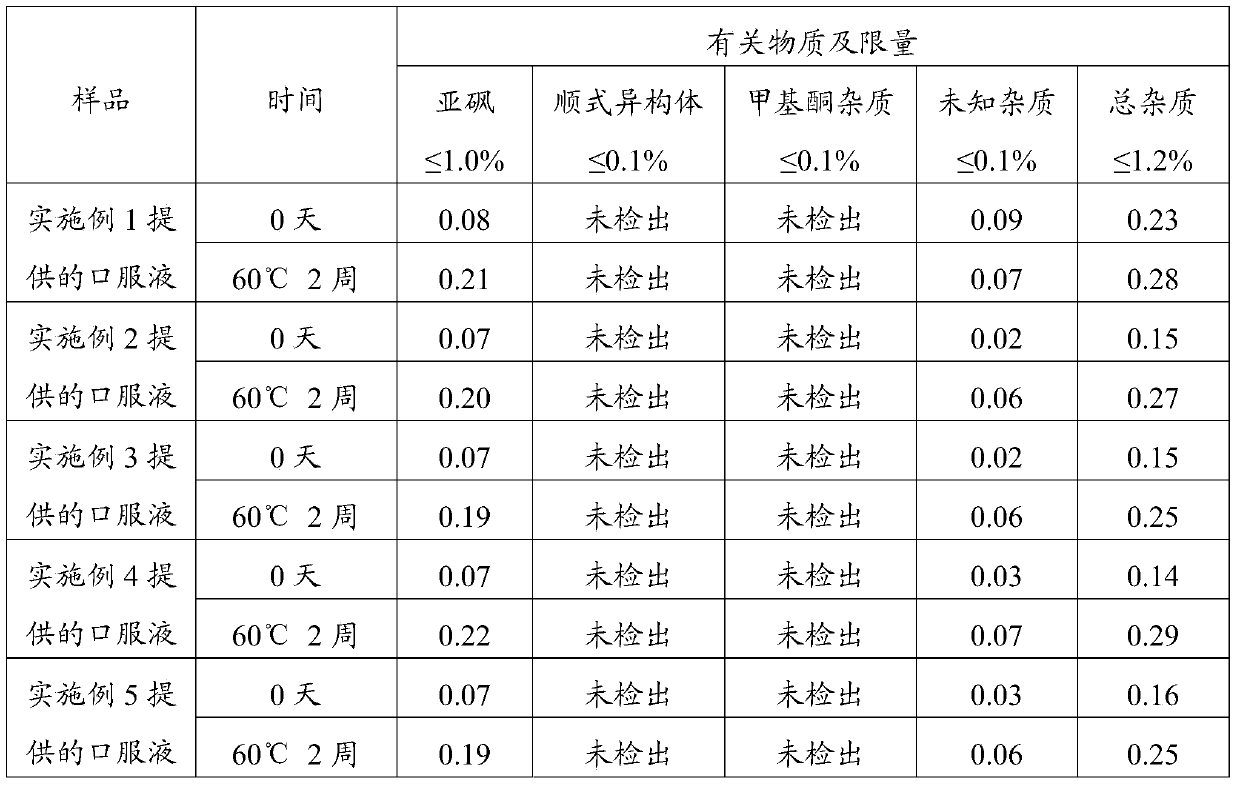
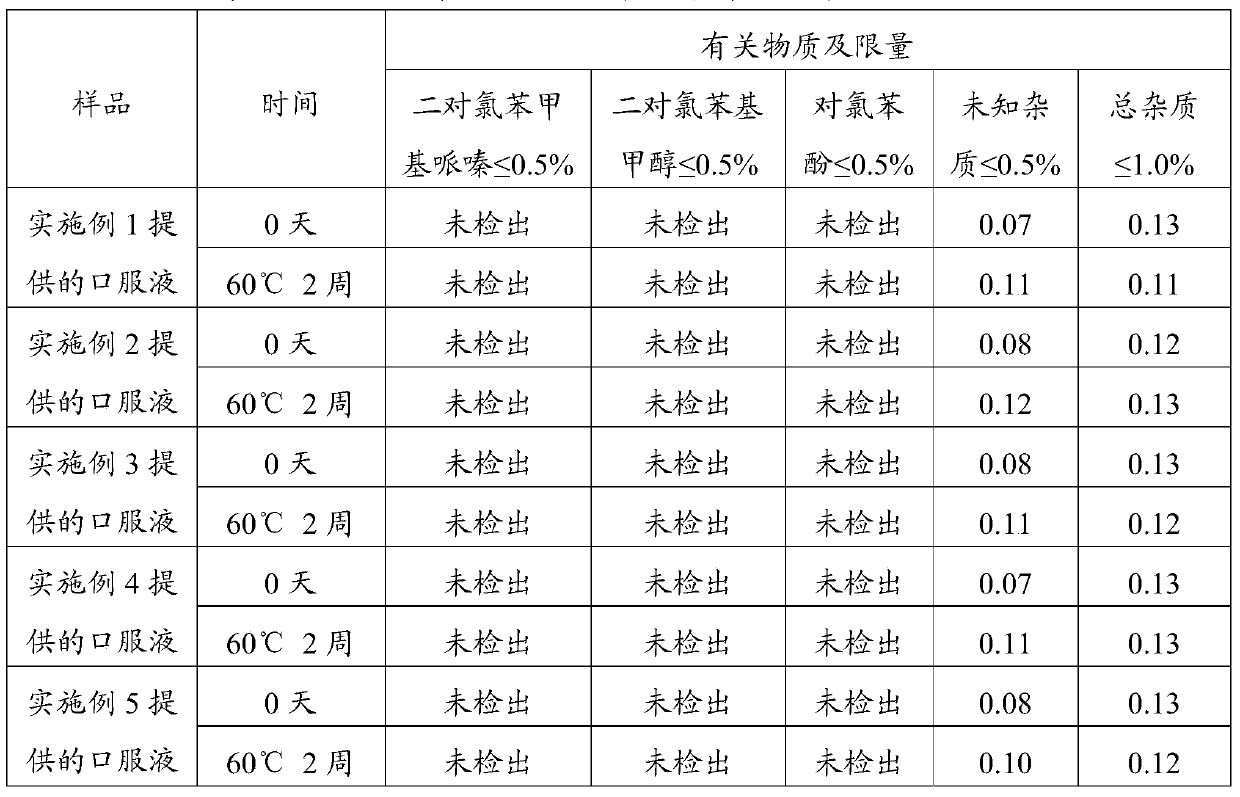
Embodiment 1
[0043] The preparation of embodiment 1 oral liquid
[0044] The prescription of oral liquid is shown in Table 1. All operations were performed under dark conditions.
[0045] Table 1 Prescription of Oral Solution
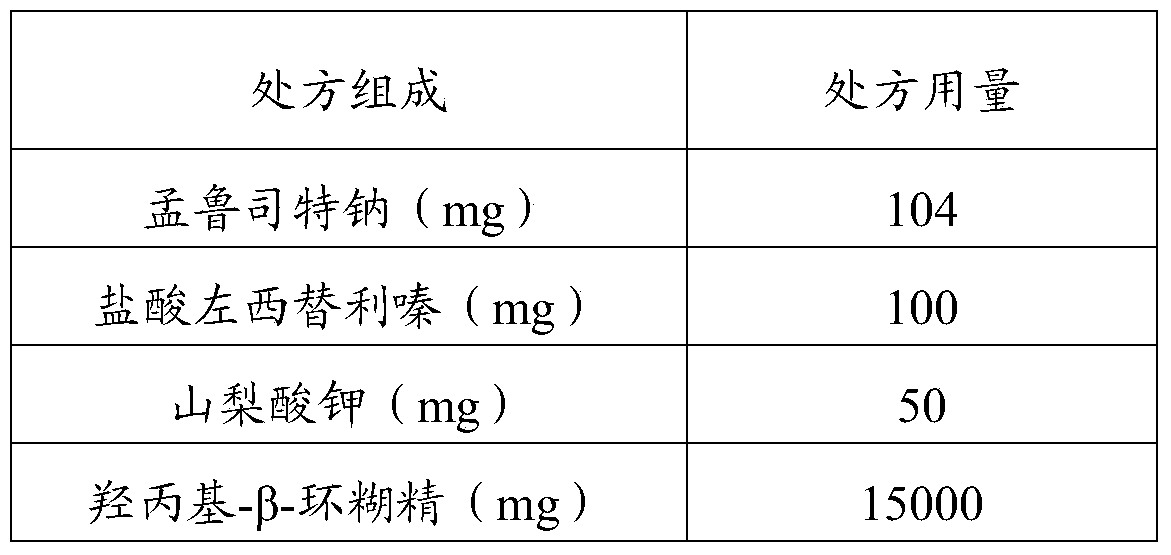
[0046] prescription composition prescription dosage Montelukast sodium (mg) 104 Levocetirizine hydrochloride (mg) 100 Meglumine (mg) 10 Hydroxypropyl-β-cyclodextrin (mg) 15000 Acesulfame K (mg) 50 Grape essence (mg) 60 Cetylpyridinium chloride (mg) 100 water (mL) 100 pH 7.38
[0047] Dissolve cetylpyridinium chloride in water, add meglumine and acesulfame potassium and stir to dissolve, add hydroxypropyl-β-cyclodextrin and stir to dissolve, add grape essence and stir to dissolve, add montelukast sodium and stir to dissolve, and obtain montelukast sodium Lukast sodium solution.
[0048] Dissolve levocetirizine hydrochloride in water, and adjust the pH to 7 with 1% sodium hydroxide to obtain levoc...
Embodiment 2
[0049] Embodiment 2 preparation of oral liquid
[0050] The prescription of oral liquid is as shown in table 2. All operations were performed under dark conditions.
[0051] Table 2 Prescription of Oral Solution
[0052]
[0053]
[0054] Dissolve cetylpyridinium chloride in water, add potassium sorbate and acesulfame potassium and stir to dissolve, add hydroxypropyl-β-cyclodextrin and stir to dissolve, add grape essence and stir to dissolve, add montelukast sodium and stir to dissolve, and obtain montelukast sodium Lukast sodium solution.
[0055] Dissolve levocetirizine hydrochloride in water and adjust the pH to 6.5 with 1% sodium hydroxide to obtain levocetirizine hydrochloride solution. Add levocetirizine hydrochloride solution to the above-mentioned montelukast sodium solution, and stir while adding to obtain the product.
Embodiment 3
[0056] Embodiment 3 preparation of oral liquid
[0057] The prescription of oral liquid is as shown in table 3. All operations were performed under dark conditions.
[0058] Table 3 Prescription of Oral Solution
[0059] prescription composition prescription dosage Montelukast sodium (mg) 50 Levocetirizine hydrochloride (mg) 500 Meglumine (mg) 40 Hydroxypropyl-β-cyclodextrin (mg) 20000 Acesulfame K (mg) 10 Grape essence (mg) 200 Cetylpyridinium chloride (mg) 200 water (mL) 100 pH 7.92
[0060] [0060] Dissolve cetylpyridinium chloride in water, add meglumine and acesulfame potassium and stir to dissolve, add hydroxypropyl-β-cyclodextrin and stir to dissolve, add grape essence and stir to dissolve, add montelukast sodium and stir to dissolve, and obtain montelukast sodium Lukast sodium solution.
[0061] Dissolve levocetirizine hydrochloride in water, and adjust the pH to 8 with 1% sodium hydroxide to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com