New high-yield preparation method of azoxystrobin
A kind of azoxystrobin, high-yield technology, applied in the field of new high-yield preparation of azoxystrobin, can solve the problems of high production cost, low yield of front intermediates, low total yield of azoxystrobin, etc., to achieve production cost The effect of reducing and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
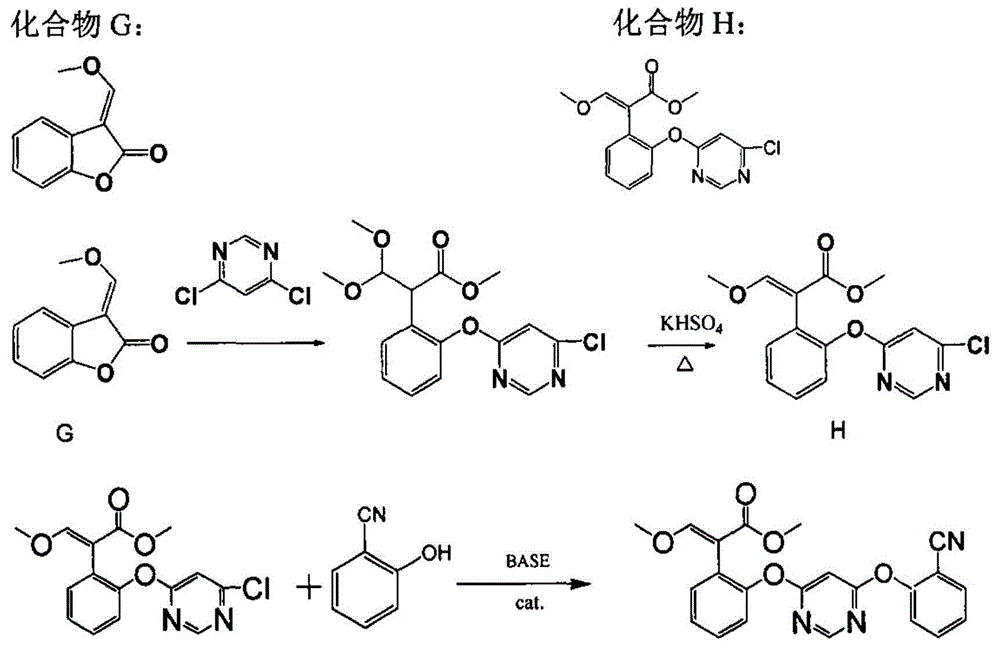
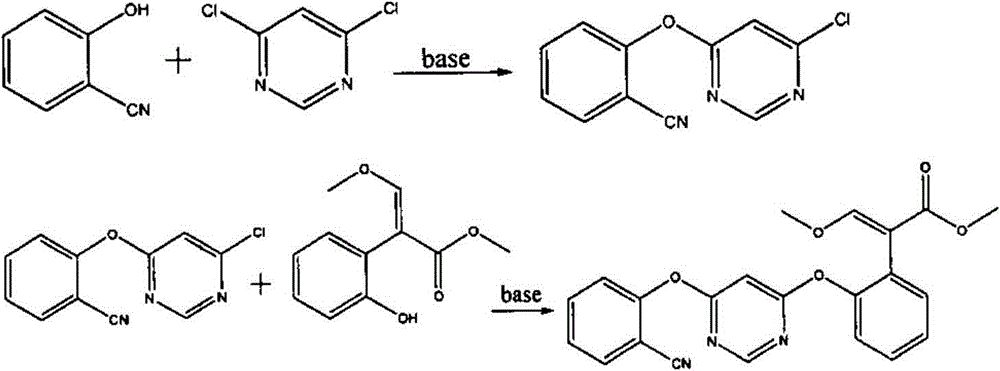
[0029] (1) Preparation of 4-chloro-6-(2-cyanophenoxy)-pyrimidine
[0030] Drop into 45.6 gram 4,6-dichloropyrimidine (98%, 0.3mol), 39.5 gram salicylonitrile (95%, 0.315mol), 13.1 gram sodium hydroxide (96%, 0.314mol) and 350 ml of toluene, stirred evenly at room temperature, then 1.1 g of 2-methyldivinylpiperazine was added, stirred and heated to 60°C for 3 hours, then heated to 80-85°C for another 1 hour. Cool down, filter, add water, stir and wash twice, remove the solvent, add methanol to recrystallize, and obtain 67.2 grams of off-white 4-chloro-6-(2-cyanophenoxy)-pyrimidine crystals (purity 98.5%, 0.287mol), Yield 95.5%.
[0031] (2) Preparation of azoxystrobin
[0032]58.6 grams of 4-chloro-6-(2-cyanophenoxy)-pyrimidine (98.5%, 0.25mol), 63.2 grams of (E)-3-methoxy-2-(2 -Hydroxyphenyl)-methyl acrylate (95%, 0.25mol), 19.6 grams of sodium hydroxide (96%, 0.47mol) and 300 milliliters of toluene, stirred at room temperature, then dropped into 0.8 grams of 2-methyldiviny...
Embodiment 2
[0034] One-pot preparation of azoxystrobin
[0035] Drop into 45.6 gram 4,6-dichloropyrimidine (98%, 0.3mol), 39.5 gram salicylonitrile (95%, 0.315mol), 13.1 gram sodium hydroxide (96%, 0.314mol) and 350 ml of toluene, stirred evenly at room temperature, then 1.1 g of 2-methyldivinylpiperazine was added, stirred and heated to 60°C for 3 hours, then heated to 80-85°C for another 1 hour. Cool down, filter, and return the filtrate to a three-necked flask, add 72.9 grams of (E)-3-methoxy-2-(2-hydroxyphenyl)-methyl acrylate (95%, 0.30mol), 23.3 grams of sodium hydroxide (96%, 0.56mol), stirred evenly at room temperature, then added 0.5 g of 2-methyldivinylpiperazine, stirred and heated to 85-90° C. for 8 hours. Cool, add water, stir and wash twice, remove the solvent, recrystallize the crude product with methanol, and finally obtain 99.7 g of light yellow azoxystrobin crystals (purity 98.2%, 0.243 mol), yield 81.0%.
Embodiment 3
[0037] A new high-yield method for preparing azoxystrobin, using a reverse two-step etherification reaction to synthesize azoxystrobin, specifically comprising the following steps:
[0038] (1) by o-hydroxybenzonitrile (compound B) and 4,6-dichloropyrimidine (compound C) in the presence of catalyst 2-methyldivinylpiperazine in the solvent toluene to carry out etherification earlier to obtain 4-chloro- 6-(2-cyanophenoxy)-pyrimidine (compound D); wherein the molar ratio of compound B, compound C and catalyst is 1:0.9:0.0005; the reaction temperature is 0°C;
[0039] (2) Carry out etherification by compound D and (E)-3-methoxy-2-(2-hydroxyphenyl)-methyl acrylate (compound E) in the presence of catalyst 2-methyldivinylpiperazine Azoxystrobin was obtained by the reaction; the molar ratio of the compound D, the compound E and the catalyst was 1:0.9:0.0005; the reaction temperature was 0°C; the obtained azoxystrobin was purified by recrystallization in the solvent methanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com