Compound based on carbazole derivative
A technology of carbazole derivatives and compounds, applied in the field of organic electroluminescent materials, can solve problems such as lower device efficiency, difficult electron flow, and charge imbalance in the light-emitting layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The synthesis of embodiment 1 compound 18
[0066]
[0067] Synthesis of Intermediate 18-1
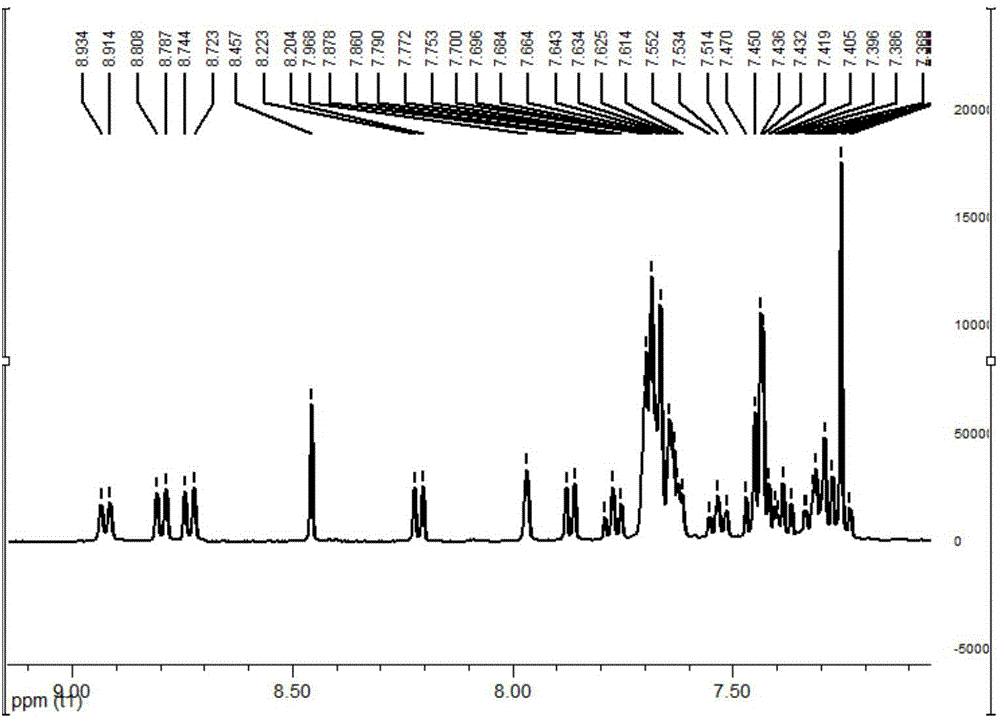
[0068] In the there-necked flask, add m-bromobenzaldehyde (5.5g), phenanthrenequinone (6g), aniline (4.5g), ammonium acetate (8g) and acetic acid (100ml), under nitrogen protection, reflux 12 hours, cool, add water, It was extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and the crude product was recrystallized with ethanol and dichloromethane to obtain 8.5 g of the product with a yield of 65%. 1 H NMR (400MHz, CDCl 3 ):δ8.46-8.86(d, J=7.6Hz, 1H), 8.76-8.78(d, J=8.4Hz, 1H), 8.70-8.72(d, J=8.4Hz, 1H), 7.83(s, 1H),7.10-7.77(m,13H).
[0069] Synthesis of Intermediate 18-2
[0070] Add compound 18-1 (4.49g, 10mmol), diboronic acid pinacol ester (3.0g, 12mmol), potassium acetate (3.0g), dichloroditriphenylphosphine palladium (50mg), dioxygen Hexacyclic (60mL), heated to 100°C for 12h, passed through silica gel while hot, washed the filter cak...
Embodiment 2
[0079] The synthesis of embodiment 2 compound 19
[0080]
[0081] Synthesis of Intermediate 19-1
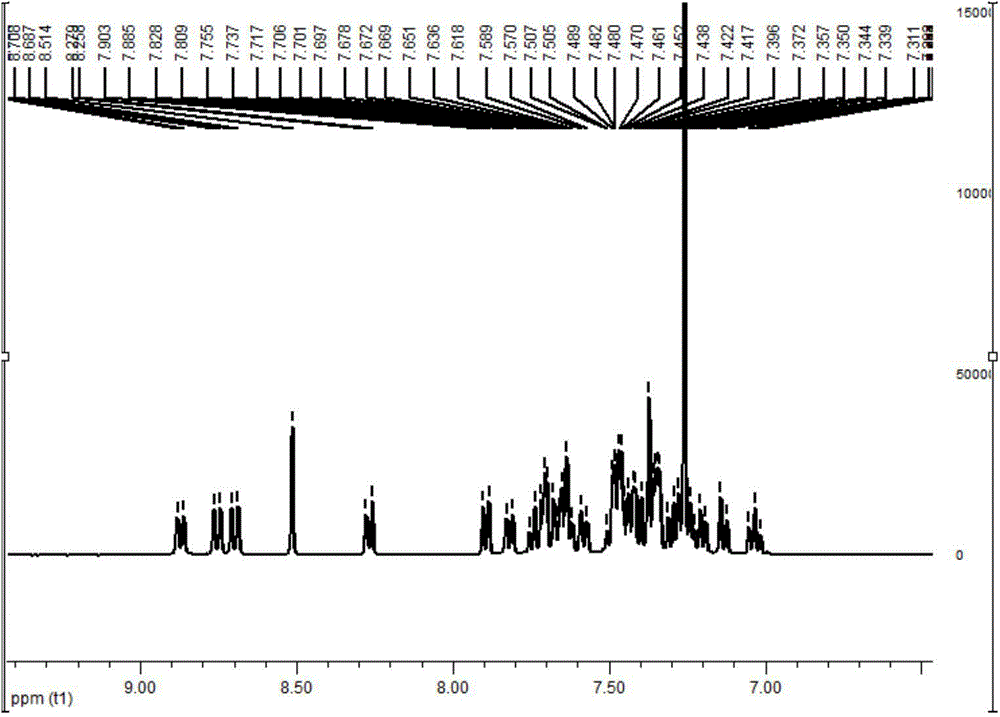
[0082] Add intermediate 18-4 (10g, 35.3mmol), m-bromoiodobenzene (12g, 42mmol), potassium tert-butoxide (7.9g, 70.6mmol), palladium acetate (0.3g, 1.3mmol), three Tert-butylphosphine tetrafluoroborate (0.8g, 2.7mmol) and toluene (150mL), heated to reflux for 24 hours under nitrogen protection, cooled, removed toluene, added dichloromethane, washed with water, dried, and the crude product was passed through the column to obtain 11 g of product, 71% yield. 1 H NMR (400MHz, CDCl 3 ):δ8.43(s,1H),8.18-8.20(d,J=7.6Hz,1H),7.85-7.87(d,J=7.6Hz,1H),7.83-7.88(m,1H),7.64- 7.66(m,1H),7.28-7.59(m,9H),1.52(s,6H).
[0083] Synthesis of Compound 19
[0084] Add intermediate 19-1 (2.1g, 4.8mmol), intermediate 18-2 (2.4g, 4.8mmol), potassium carbonate (3g), tetrakistriphenylphosphine palladium (30mg), tetrahydrofuran (20mL) in a single-necked flask ), water (11 mL), heated to 80 ° C for 2...
Embodiment 3
[0086] Fabrication of Organic Electroluminescent Devices
[0087] Preparation of OLED using compound 19 of Example 2
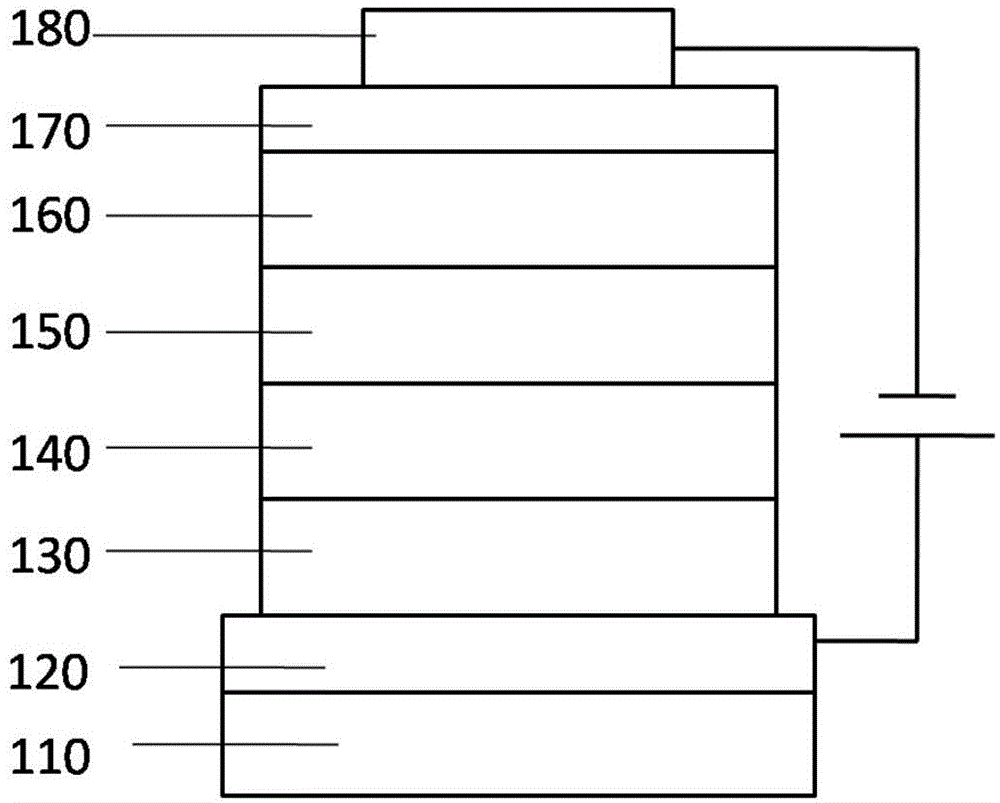
[0088] First, the transparent conductive ITO glass substrate 110 (with the anode 120 on it) (China CSG Group Co., Ltd.) is washed with deionized water, ethanol, acetone and deionized water in sequence, and then treated with oxygen plasma for 30 seconds.
[0089] Then, a 5nm thick HAT-CN is vapor-deposited on the ITO as the hole injection layer 130
[0090] Then, TAPC was evaporated to form a hole transport layer 140 with a thickness of 65 nm.
[0091] Then, a 10nm-thick light-emitting layer 150 was evaporated on the hole-transport layer, in which compound 19 was used as the host light-emitting material, and 7% by weight Ir(ppy)2acac was used as the phosphorescence-doped guest material.
[0092] Then, 50 nm thick TmPyPB was vapor-deposited on the light emitting layer as the electron transport layer 160 .
[0093] Finally, 1nm LiF was vapor-deposited as the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com