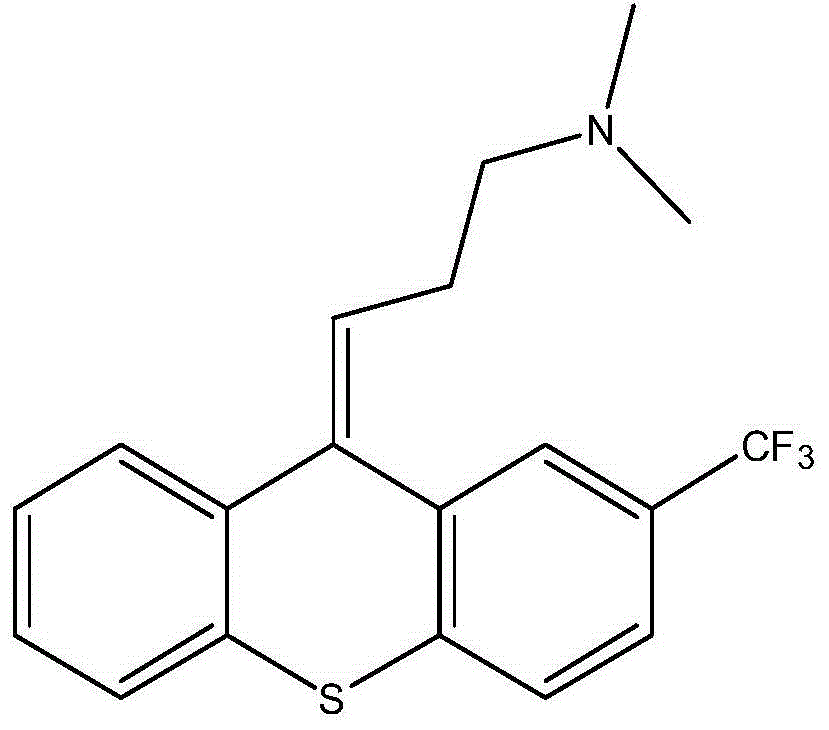
Preparation method of flupentixol dihydrochloride
A technology of flupentixol hydrochloride and flupentixol hydrochloride is applied in the field of preparation of flupentixol hydrochloride, can solve the problems of inconvenient industrialized production, low yield, complicated procedures and the like, and achieves few steps, high yield, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: a kind of embodiment of the preparation method of 10-[3-(EZ)-dimethylaminopropyl group]-2-trifluoromethylthioxanthene of the present invention (acetic anhydride and 10-hydroxyl-10 The molar ratio of -(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene is about 1.46:1)
[0027] Put 3.67kg of 10-hydroxy-10-(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene and 35kg of tetrahydrofuran into the reaction kettle, add 1.7kg of acetic anhydride at room temperature for reaction, and concentrate under reduced pressure after the reaction is completed The reaction solution, the residue was dissolved in 40 kg of ethyl acetate, then washed with 40 kg of saturated sodium bicarbonate solution, the ethyl acetate layer was dried and concentrated under reduced pressure to obtain 10-[3-(EZ)-dimethylaminopropyl]- 2-trifluoromethylthioxanthene, its Z-type product content is 48%, yield: 100%. 10-[3-(EZ)-dimethylaminopropyl]-2-trifluoromethylthioxanthene Z type content dete...
Embodiment 2
[0028] Embodiment 2: a kind of embodiment of the preparation method of 10-[3-(EZ)-dimethylaminopropyl group]-2-trifluoromethylthioxanthene of the present invention (acetic anhydride and 10-hydroxyl-10 The molar ratio of -(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene is 1:1)
[0029] Put 3.22kg of 10-hydroxy-10-(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene and 35kg of acetone into the reaction kettle, add 1.02kg of acetic anhydride at room temperature for reaction, and concentrate under reduced pressure after the reaction is completed The reaction solution, the residue was dissolved in 40 kg of ethyl acetate, then washed with 40 kg of saturated sodium bicarbonate solution, the ethyl acetate layer was dried and concentrated under reduced pressure to obtain 10-[3-(EZ)-dimethylaminopropyl]- 2-trifluoromethylthioxanthene, its Z-type product content is 46%, yield: 100%. 10-[3-(EZ)-dimethylaminopropyl]-2-trifluoromethylthioxanthene Z type content determination method...
Embodiment 3
[0030] Embodiment 3: a kind of embodiment of the preparation method of 10-[3-(EZ)-dimethylaminopropyl group]-2-trifluoromethylthioxanthene of the present invention (acetic anhydride and 10-hydroxyl-10 The molar ratio of -(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene is 4:1)
[0031]Put 3.22kg of 10-hydroxy-10-(3-dimethylaminopropyl)-2-trifluoromethylthioxanthene and 35kg of tetrahydrofuran into the reaction kettle, add 4.08kg of acetic anhydride at room temperature for reaction, and concentrate under reduced pressure after the reaction is completed The reaction solution, the residue was dissolved in 40 kg of ethyl acetate, then washed with 40 kg of saturated sodium bicarbonate solution, the ethyl acetate layer was dried and concentrated under reduced pressure to obtain 10-[3-(EZ)-dimethylaminopropyl]- 2-trifluoromethylthioxanthene, its Z-type product content is 49%, yield: 100%. 10-[3-(EZ)-dimethylaminopropyl]-2-trifluoromethylthioxanthene Z type content determination...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com