Preparation method of composite metal oxide and synthesis method of alcohol ether carboxylate
A technology of alcohol ether carboxylate and composite metal, which is applied in the preparation of composite metal oxide and the field of composite metal oxide catalyzed synthesis of alcohol ether carboxylate, can solve the problem of product limitation and the like, so as to improve the reaction activity and catalytic activity Enhanced, reduced aggregation time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Catalyst Preparation
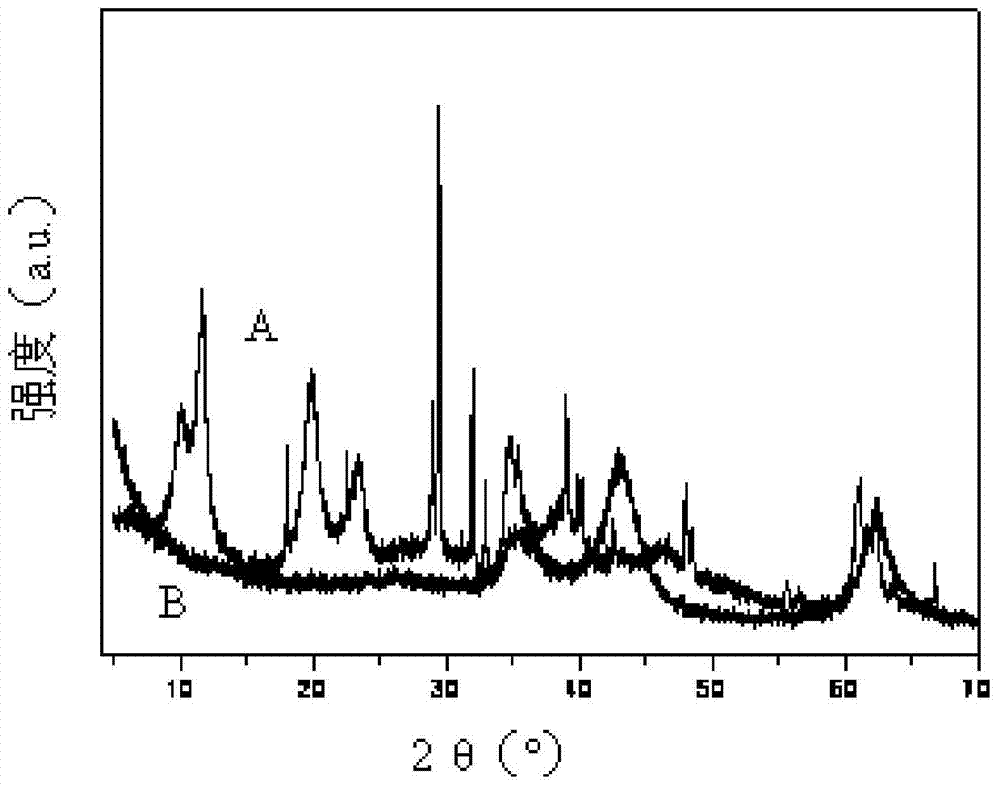
[0042] Weigh 192 grams of Mg(NO 3 ) 2 ·6H 2 O crystals and 141 g of Al(NO 3 ) 3 9H 2 O crystals were dissolved in 1 liter of deionized water to form Al(NO 3 ) 3 and Mg(NO 3 ) 2 mixed solution. Put 0.9mol / L Na 2 CO 3 300ml of the solution was added to the above mixed solution. Add 2.25mol / L NaOH solution dropwise into the mixed solution to adjust the pH value of the solution to 10±0.5. Continue to stir the mixed solution at room temperature for about 5-6 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight. For the XRD spectrum of the sample, see figure 1 Figure A.
[0043] Take 10 grams of the dried sample, add it to 400 mL of 0.0025 g / mL lauric acid in isopropanol solution, boil and reflux for about 2 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight. The XRD spectrum pattern of the compound obtained, see figure 1 Figure B. Finally, it was calcined a...
Embodiment 2
[0051] 1. Preparation of composite metal oxide catalysts
[0052] Weigh 192 grams of Mg(NO 3 ) 2 ·6H 2 O crystals, and 47 grams of Al(NO 3 ) 3 9H 2 O crystals were dissolved in 1 liter of deionized water to form Al(NO 3 ) 3 and Mg(NO 3 ) 2 mixed solution. Put 0.9mol / LNa 2 CO 3 Add 300mL of the solution into the above mixed solution, and then add 2.25mol / L NaOH solution dropwise into the mixed solution to adjust the pH value of the solution to 10±0.5. Continue to stir the mixed solution at room temperature for about 5-6 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight.
[0053] Take 10 grams of the dried sample, add it to 500 mL of 0.002 g / mL stearic acid in isopropanol solution, boil and reflux for about 4 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight . Finally, it was calcined at 250° C. for 5 hours in a muffle furnace to obtain the final composite metal oxide catalyst.
[0054...
Embodiment 3
[0061] 1. Catalyst preparation
[0062] Weigh 77g Mg(NO 3 ) 2 ·6H 2 O crystals, 19g Al(NO 3 ) 3 9H 2 O crystals and 41 g FeCl 3 ·6H 2 O was dissolved in 1 liter of deionized water to form Al(NO 3 ) 3 , Mg(NO 3 ) 2 and FeCl 3 mixed solution. Put 0.9mol / L Na 2 CO 3300ml of the solution was added to the above mixed solution. Add 2.25mol / L NaOH solution dropwise into the mixed solution to adjust the pH value of the solution to 10±0.5. Continue to stir the mixed solution at room temperature for about 5-6 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight.
[0063] Take 10 grams of the dried sample, add it to 400 mL of 0.0025 g / mL lauric acid in isopropanol solution, boil and reflux for about 2 hours, then filter with suction, wash until neutral, and dry in an oven at 80°C overnight. Finally, it was calcined at 300° C. for 5 hours in a muffle furnace to obtain the final composite metal oxide catalyst.
[0064] 2. Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com