Method for preparing terbinafine hydrochloride Z-shaped isomer
A technology of terbinafine hydrochloride and terbinafine, which is applied in the field of medicine, can solve the problems of undocumented impurity analysis, detection and the like, and achieve the effects of stable product quality, mild preparation conditions and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
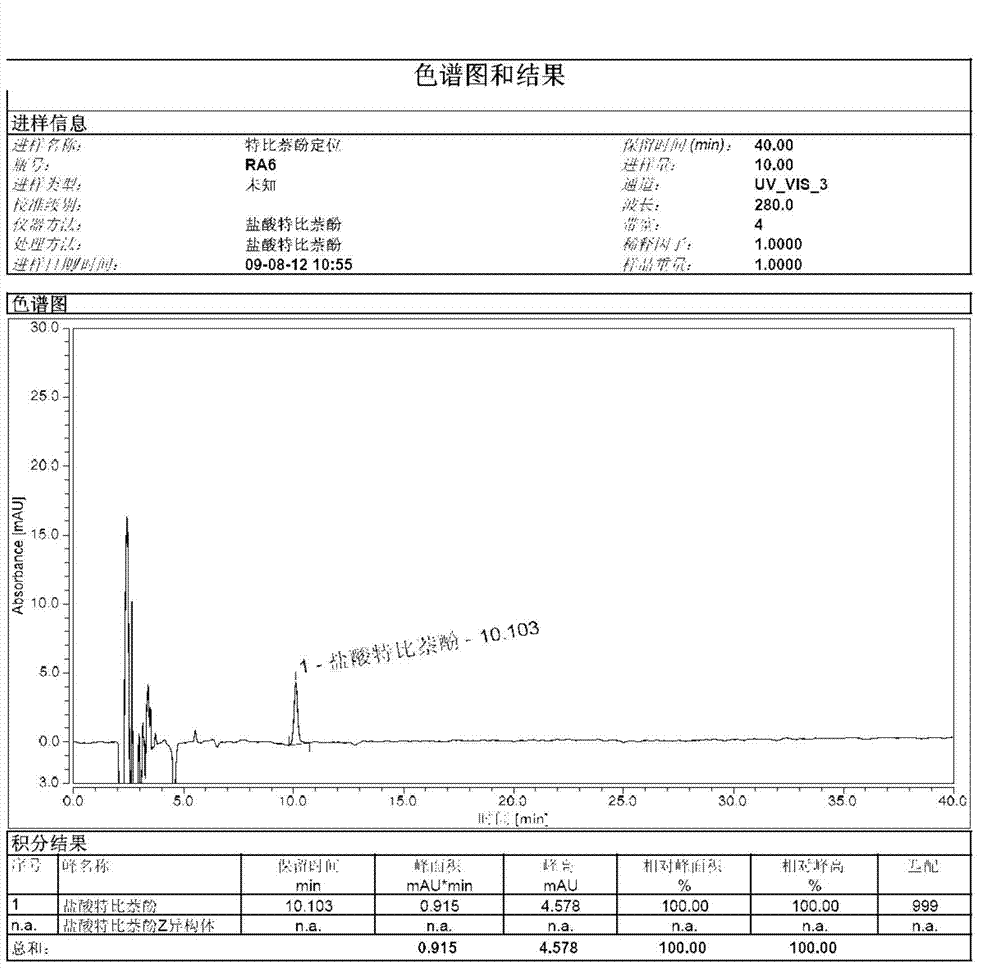
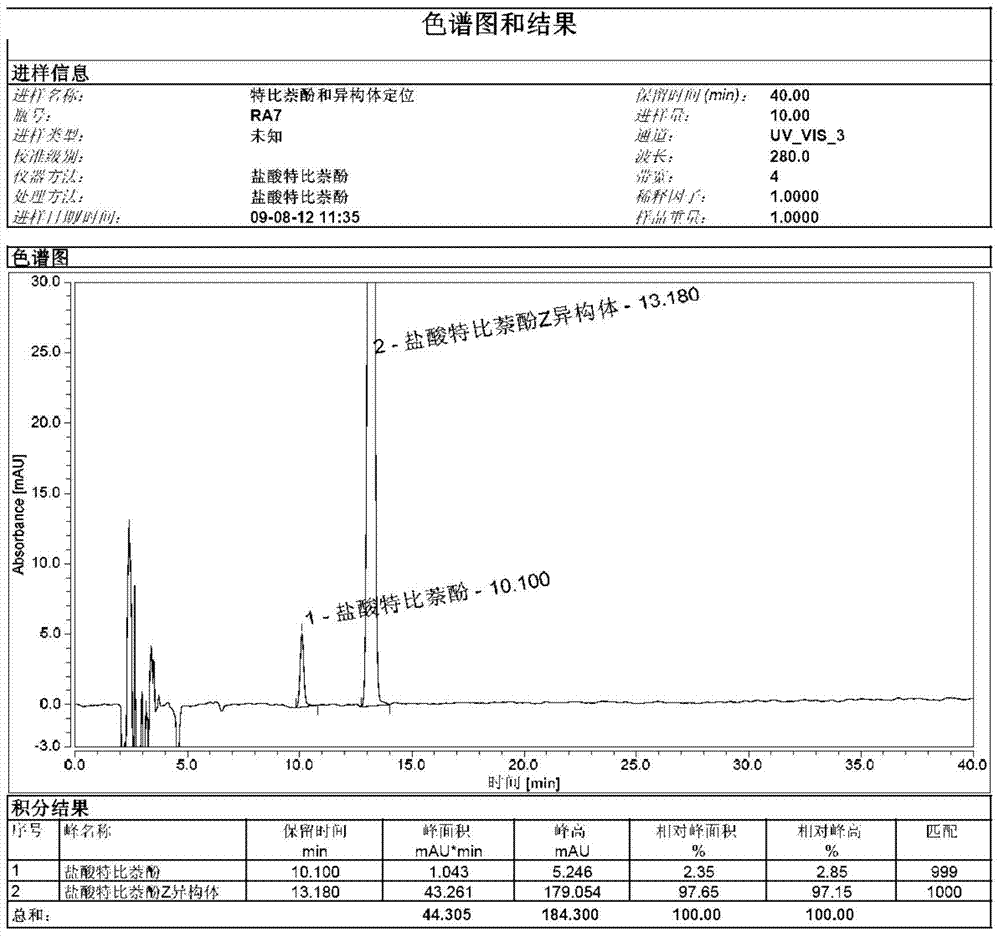
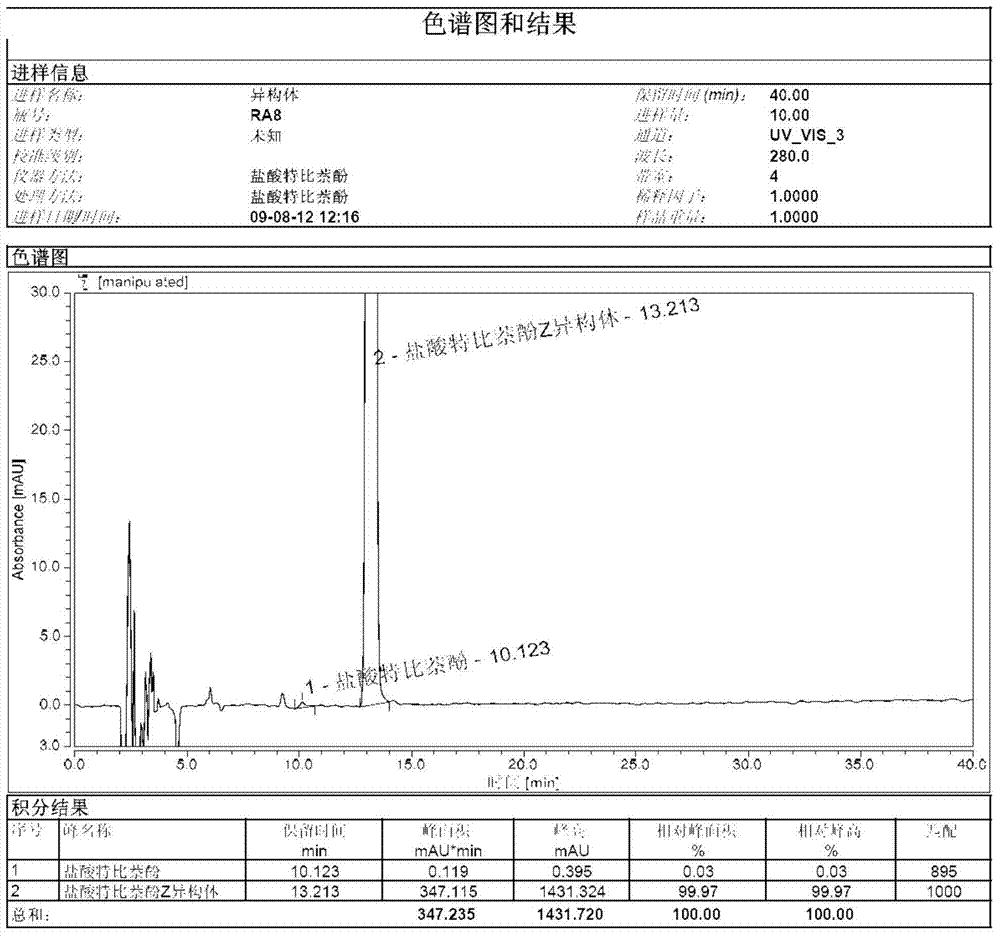
[0018] Weigh 50.0 g of terbinafine EZ mixture into a 500 ml three-necked flask, add 200 ml of isopropanol to it, raise the temperature to 70° C., and keep it warm for 2 hours. Slowly lower the temperature to 20 degrees Celsius and keep it for 2 hours, then lower the temperature to 0 degrees Celsius and keep it for 2 hours. After filtration, the filtrate was concentrated under reduced pressure to obtain an oil.
[0019] Put 10g of oil in a 100ml three-necked flask, add 50ml of 4-chlorophenyl tert-butyl ether, increase the temperature to 120°C, and keep it warm for 1 hour. Then, the temperature was lowered to 10°C, kept for 3 hours, and filtered to obtain a white compound, terbinafine hydrochloride Z isomer. 1 H-NMR (400MHz, CDCl 3 )δ1.25(s, 9H), 2.27(d, 2H), 3.03(d, 2H), 4.06(d, 2H), 5.62(t, 1H), 6.21(t, 1H), 7.10(m, 1H) ), 7.19(m, 1H), 7.29(m, 1H), 7.31(m, 1H), 7.51(m, 1H), 7.64(m, 1H), 7.77(m, 1H)
[0020] Use octadecyl silane-bonded silica gel as a filler (150mm×3.0mm, 5μm, or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com