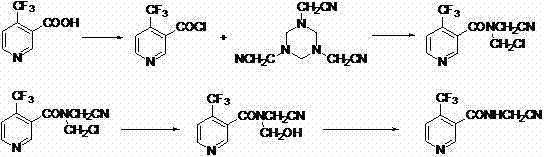
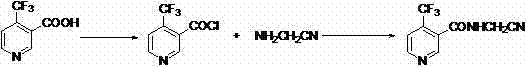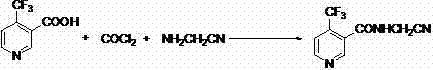A synthetic method of N-cyanomethyl-4-(trifluoromethyl)nicotinamide
The technology of a trifluoromethyl group and a synthetic method, applied in the field of pesticides, can solve the problems of high production safety risks and the like, and achieve the effects of convenient operation, simple process and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] After adding 19g (0.1mol) of 4-(trifluoromethyl)nicotinamide, 12.72g (0.12mol) of sodium carbonate and 80g of dimethylformamide in a 250mL reactor with a reflux condenser, at 15± 8.25 g (0.11 mol) of chloroacetonitrile was added dropwise with stirring at 5°C, and then the reaction temperature was raised to 80°C for 8 hours.
[0028] After the reaction, the liquid layer was analyzed by liquid chromatography, and the result was that the yield (selectivity) of N-cyanomethyl-4-(trifluoromethyl)nicotinamide was 96.8%, and 4-(trifluoromethyl) ) The conversion rate of nicotinamide is 98.9%, and the yield is selectivity, which is the converted yield without separation and purification.
Embodiment 2
[0030] After adding 19g (0.1mol) of 4-(trifluoromethyl)nicotinamide, 15.9g (0.15mol) of sodium carbonate and 100g of dimethylformamide in a 250mL reactor with a reflux condenser, at 15± 8.25 g (0.11 mol) of chloroacetonitrile was added dropwise with stirring at 5°C, and then the reaction temperature was raised to 80°C for 8 hours.
[0031] After the reaction, the liquid layer was analyzed by liquid chromatography, and the result was that the yield (selectivity) of N-cyanomethyl-4-(trifluoromethyl)nicotinamide was 96.5%, and 4-(trifluoromethyl) ) The conversion rate of nicotinamide is 98.6%, and the yield is selectivity, which is the converted yield without separation and purification.
Embodiment 3
[0033] After adding 19g (0.1mol) of 4-(trifluoromethyl)nicotinamide, 15.9g (0.15mol) of sodium carbonate and 100g of dimethylformamide in a 250mL reactor with a reflux condenser, at 15± 11.25 g (0.15 mol) of chloroacetonitrile was added dropwise with stirring at 5°C, and then the reaction temperature was raised to 80°C for 8 hours.
[0034] After the reaction, the liquid layer was analyzed by liquid chromatography, and the result was that the yield (selectivity) of N-cyanomethyl-4-(trifluoromethyl)nicotinamide was 96.2%, and 4-(trifluoromethyl) ) The conversion rate of nicotinamide is 98.1%, and the yield is selectivity, which is the converted yield without separation and purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com