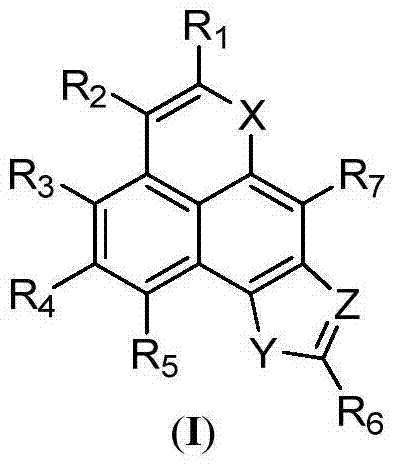
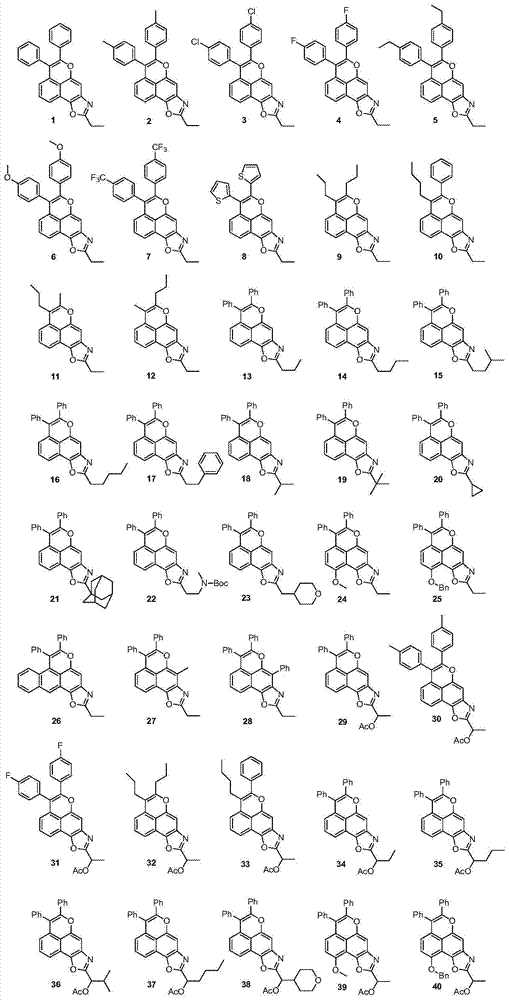
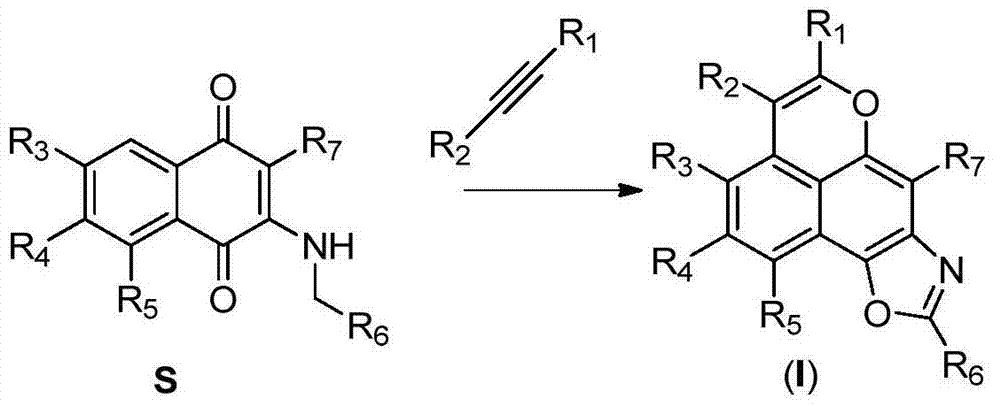
Novel tetracyclonaphthooxazole derivative and preparation method thereof
A technology of naphthooxazole derivatives and derivatives, which is applied in the field of novel tetracyclic naphthooxazole derivatives and their preparation, and can solve the problems of limiting the application of such compounds, cumbersome synthesis methods, and low synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] The preparation of 2-substituted aminoquinone substrates refers to Khodade, V.S.; Dharmaraja, A.T.; Chakrapani, H.Bioorg.Med.Chem.Lett.2012,22,3766.
[0051] Weigh 86mg (0.4mmol) 2-n-propylamino-1,4-naphthoquinone, 107mg (0.6mmol) diphenylacetylene, 12mg (0.02mmol) [Rh(Cp*)Cl 2 ] 2 (Cp*=pentamethylcyclopentadiene), 12mg (0.04mmol) AgSbF 6 and 160 mg (0.8 mmol) Cu(OAc) 2 .H 2 O was added to a round bottom reaction flask, dissolved with 2mL t-AmOH, and heated at 120°C for 6h to complete the reaction. Added 10 mL of water, extracted three times with ethyl acetate (5 mL x3), combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated the solvent under reduced pressure, and separated by silica gel column chromatography to obtain 113 mg of light yellow solid compound 1, Yield 73%; 25mg light yellow solid compound 29, yield 14%.
[0052] Compound 1:
[0053] 1 H NMR (300MHz, CDCl 3 )δ7.60(d,J=8.4Hz,1H),7.33(m,5H),...
Embodiment 2
[0057]
[0058] Except that diphenylacetylene was replaced by di-p-methylphenylacetylene, 110 mg of light yellow solid compound 2 was prepared according to the method of Example 1, and the yield was 66%. 27 mg of light yellow solid compound 30, yield 14%.
[0059] Compound 2:
[0060] 1 H NMR (400MHz, CDCl 3 )δ7.62(d,J=8.3Hz,1H),7.27(m,5H),7.17(m,3H),7.03(d,J=8.1Hz,2H),6.53(m,1H),3.06( q,J=7.6Hz,2H),2.42(s,3H),2.32(s,3H),1.53(t,J=7.6Hz,3H); 13 C NMR (101MHz, CDCl 3 )δ168.1, 150.0, 149.9, 140.5, 138.5, 138.4, 137.1, 133.5, 132.3, 131.2, 130.9, 129.8, 128.8, 128.6, 128.4, 120.5, 120.0, 115.8, 115.6, 98.3, 21.3 (2) EI-MS (m / z) 417 (M + ), HRMS C 29 h 23 NO 2 [M + ], calculated value: 417.1729, measured value: 417.1728.
[0061] Compound 30:
[0062] 1 H NMR (300MHz, CDCl 3 )δ7.63(dd,J=8.3,0.9Hz,1H),7.29(dd,J=8.2,7.5Hz,1H),7.16(m,7H),7.00(d,J=7.9Hz,2H), 6.54(dd, J=7.4,0.9Hz,1H),6.18(q,J=6.7Hz,1H),2.38(s,3H),2.28(s,3H),2.18(s,3H),1.80(d ,J=6.7Hz,3H); 13 C NMR (1...
Embodiment 3
[0064]
[0065] Except that diphenylacetylene was replaced by di-p-chlorophenylacetylene, 117 mg of light yellow solid compound 3 was prepared according to the method of Example 1, and the yield was 64%.
[0066] 1 H NMR (300MHz, CDCl 3 )δ7.61(d,J=8.4Hz,1H),7.36(d,J=8.3Hz,2H),7.24(m,2H),7.18(m,4H),7.14(m,2H),6.43( d,J=7.4Hz,1H),3.02(q,J=7.6Hz,2H),1.49(t,J=7.6Hz,3H); 13 C NMR (101MHz, CDCl 3 )δ168.3, 149.5, 149.1, 140.7, 138.6, 134.7, 133.8, 133.5, 132.4, 132.1, 130.2, 129.6, 128.6, 128.1, 120.3, 120.0, 116.6, 115.7, 115.5, 98.1-MS, 22.3 / z)457(M + ), HRMS C 27 h 17 NO 2 Cl 2 [M + ], calculated value: 457.0636, measured value: 457.0633.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com